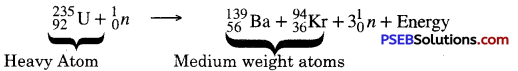Punjab State Board PSEB 10th Class Science Important Questions Chapter 14 Sources of Energy Important Questions and Answers.
PSEB 10th Class Science Important Questions Chapter 14 Sources of Energy
Long Answer Type Questions
Question 1.
Draw a labelled diagram of a Box Type Solar Cooker. Also give its working and advantages.
Answer:
Construction: Box type solar cooker consists of an insulated box (wooden or plastic) which is painted black from inside. A plane mirror reflector is attached to the box (figure). The food to be cooked is placed in containers which are also painted black from outside.
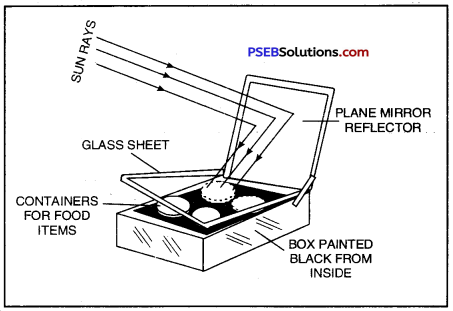
A Box Type Solar Cooker
Working: The containers with food to be cooked are placed in the box and the box is covered with thick glass sheet. The cooker is kept in the sunlight and the position of the reflector is adjusted such that a strong beam of sunlight
falls over the glass sheet. These rays pass through the transparent glass sheet cover and are absorbed by the cooker box and the containers and also heat radiations are not allowed to escape. The use of black surface of the box and containers is due to the fact that a black surface absorbs more heat than any other colour. Therefore, the box and the containers absorb maximum amount of infra-red radiations from the sunlight falling on it. As a result, the temperature inside the box rises to about 100°-140°C in two to three hours and this heat cooks the food.
Limitation of Solar Cooker: This type of solar cooker can be used to prepare only food items which require slow heating and cannot be used for baking and frying purposes.
Advantages of a Solar Cooker: The important advantages of a solar cooker are given ahead :
- The use of solar cooker for cooking food materials saves fuel.
- It is an economical method of cooking food since no fuel is used.
- It does not cause any pollution because this does not give out any smoke.
- In this method, food is cooked at comparatively lower temperature therefore, the nutrients of food do not get destroyed during cooking.
Question 2.
With the help of a labelled diagram, describe how electricity is generated in a nuclear power plant?
Answer:
Nuclear Power Plant. It consists the following parts :
1. Nuclear Reactor ‘R’: It is a device in which energy is produced by a controlled nuclear fission of U-235. The reactor R is enclosed in a concrete chamber ‘AT having thick whlls to protect the outside world from nuclear radiations.
The nuclear reaction consists of steel vessel ‘Y having graphite blocks which act as a moderator. The graphite core has number of enriched U-235 rods which act as a fuel. These are marked as ‘A’. In between the uranium rods are inserted cadmium or boron rods and which can be lowered or raised from outside. These rods will keep the fission under control.
2. Heat Exchanger ‘H’: The reactor ‘R’ is connected to a heat exchanger ‘H’ by pipes. The heat exchanger is a sort of bioler which contains water. The heat produced in the reactor is carried to water through coiled pipes.
3. Steam Turbine ‘T’: The heat exchanger is connected to a steam turbine T and its shaft is connected to an electric generator ‘G’. The electricity produced by the generator is sent for transmission.
Working. After the uranium enriched rods are placed in position, the reactor is sealed. The cadmium or boron rods in the beginning are put inside so that they may absorb all neutrons emitted by U-235 and prevent fission all at once. The controlling rods of cadium
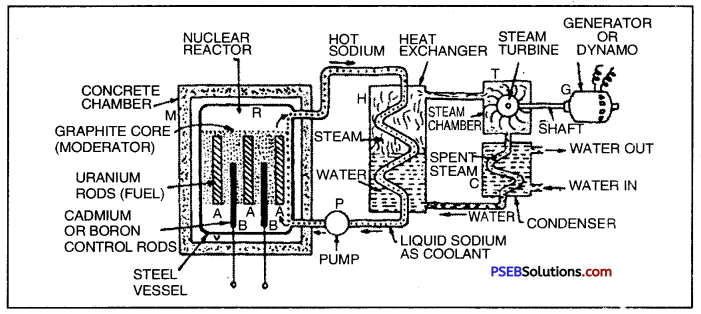
Nuclear Power Plant
or boron are withdrawn till they emit desirable number of neutrons. At this stage a controlled reaction takes place. The heat produced is withdrawn by circulating sodium. When sodium passes through the coil in the exchange it transfers heat to water. Now the water changes into steam which is passed into turbine chamber ‘T’. The turbine rotates the coil of the generator ‘G’ when electricity is generated.
Question 3.
Draw diagram of Fixed Dome Type Biogas Plant and describe how biogas can be produced with this plant?
Answer:
Fixed Dome Type Biogas Plant. The plant consists of underground tank made of bricks called digester. The roof of the digester is dome-shaped made of cement and bricks. It acts as a storage tank for biogas. In the mixing tank, cattle dung is mixed with water to prepare slurry. This slurry is then fed into the inlet chamber for onward supply to digester through inlet T. At the bottom of digester and just opposite to the inlet there is outlet ‘O’ taking spent us slurry to the outlet chamber. There is a gas outlet ‘S’ at the top of dome for the supply of bio-gas.
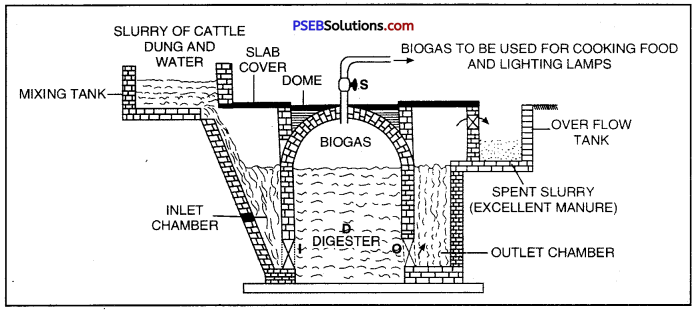
Fixed Dome Type Biogas Plant
Working of Plant: Animal waste and water are mixed in equal proportions in the mixing tank to form a slurry. The slurry is fed into the digester tank through the inlet chamber. The tank is closed for about two months. During this period, the animal waste undergoes fermentation by anaerobic bacteria. As a result of fermentation, biogas is formed and collected in the dome. As more and more biogas gets collected in the dome, it exerts pressure on the slurry in the digestion tank and forces it to go into the over flow tank through the outlet chamber.
The spent slurry being rich in nitrogen and phosphorus can be used as a manure. Whenever required the biogas collected in the dome can be taken out through a pipe ‘S’ Once the biogas plant starts functioning, more and more slurry may be fed into the digester to get a continuous supply of the biogas.

Question 4.
Explain the working of Floating Gas Holder Type Biogas Plant with the help of a neat labelled diagram.
Answer:
Construction of Floating Gas Holder Type Biogas Plant. The plant consists an underground well shaped tank made of bricks. It is called digester. A drum shaped gasholder made of steal meant for collecting gas is kept in the inverted position floating over cattle dung slurry on the digester tank. The up and down movement of gasholder is controlled by a pipe. At the top of the gasholder there is an outlet for gas. The digester tank is divided into two parts with a partition wall. On the left side of the digester tank there is an inlet pipe. The inlet pipe is connected to the mixing tank through which slurry is allowed to get into the digester. On the right side of the digester tank there is an outlet pipe which is connected to the overflow tank.
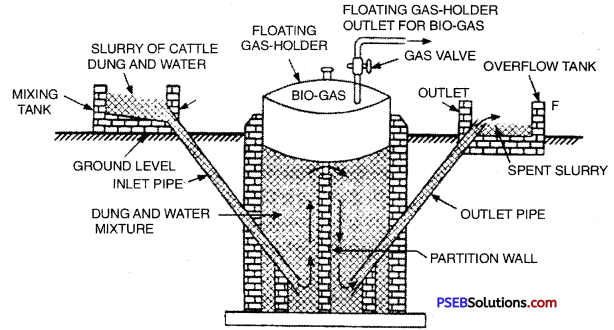
Floating Gas Holder type Bio-gas Plant
Working: Cattle dung and water are mixed in equal proportions in the mixing tank to make slurry. The slurry is then introduced into the digester tank through the inlet pipe. It is left for about 2 months during which dung undergoes anaerobic fermentation producing biogas. The biogas so produced starts collecting in the gas holder when more and more gas is produced, the gas holder starts rising. The spent up slurry left over is used as manure.
Question 5.
Describe a wind mill and give its functions?
Answer:
Wind mill. A wind-mill is a machine which works with the energy of wind and converts kinetic energy of wind into mechanical energy or electricity. It consists of large fan like blades to catch the wind. When the wind strikes against these blades, the blades start rotating. This motion is then passed onto connected crankshaft and is used to draw water from wells and to run flour mills for grinding grains. The pictures of wind-mill drawing water from tube wells. [Figure] and wind will grinding grains (Figure) are shown ahead.
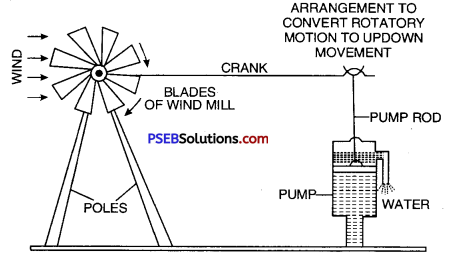
A wind-mill drawing water from the under ground.
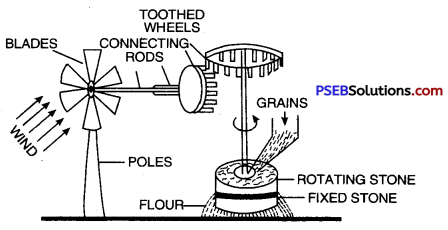
Wind mill used to grind grains.
Functions of Wind Mill.
- Wind mill is used for drawing water from tube wells.
- Wind mill for grinding grains. Windmill is being used to grind grains by suitable arrangement of toothed wheel and shaft.
Question 6.
What is a Solar Cell? What is its working principle? Write short note on the making of solar cells and also give its uses.
Answer:
Solar Cell. It is a device which converts solar energy directly into electricity.
Working Principle. Semi conductors (substances whose conductivity lies between metal and insulator) possess very low electrical conductivity. Silicon (Si), Gallium (Ga) and Germanium (Ge) are some examples of semi-conductors. In fact, semi-conductors are neither conductor nor insulator. However, it has been found that their conductivity increases when they are mixed with phosphorus or arsenic. Since the efficiency of such solar cells made of silicon and gallium was very low (10% to 15%) whereas the efficiency of modern cells is as high as 25%.
Making of Solar Cells. To make a solar cell thin wafers of semi-conducting materials containing impurities are arranged in such a way that when solar light falls on them, a potential difference develops between two areas of the semi-conductor. This potential difference results in producing current. A single solar cell produces an elective current of 60 mA (milli-amperes).
Uses of Solar Cells: The important uses of solar cells are given below :
- It is used for generating electricity for operating on board artificial sattelites and spaceprobes.
- Solar cells are used to provide electricity to “light houses” situated in the sea and off-shore oil drilling platform.
- Solar cells are used for operating electronic watches and calculators.
- Solar cells are used for street lighting, for operating water pumps and working of television in remote areas where electricity is not available.
Question 7.
What is solar cell panel? Describe with the help of a suitable diagram.
Or
How is solar panel prepared? Explain with diagram.
Answer:
Solar Cell Panel: A single solar cell consisting of thin wafer of silicon-arsenic or silicon-boron produces a very weak current. In order to get sufficiently high current so as to be capable of lifting water from deep well or to light a house, a large number of solar cells are joined together in a definite pattern to get solar cell panel.
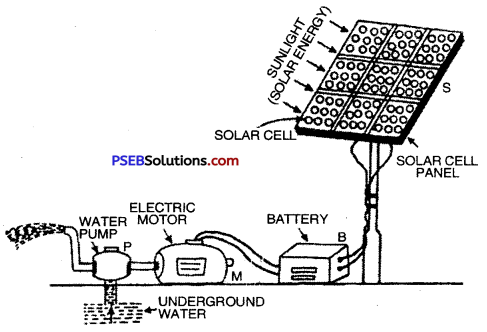
Solar cell Panel being used for operating a water pump
The above figure depicts a simple arrangement of a solar cell panel. In this solar cell panel hundreds of solar cells are joined together. The electric power produced by this solar cell panel is stored in battery B. This battery runs an electric motor M which drives water pump P to take out water from underground.
Question 8.
What is Nuclear Fission? Explain this phenomenon with the help of an example.
Answer:
Nuclear Fission: The process in which an unstable nucleus of a heavy radioactive atom (like Uranium-235) splits up into two or more fragments of medium weight nuclei when bombarded by slow moving neutrons (possessing low energy) with the release of enormous amount of energy is called Nuclear fission.
In this process there is always a small loss of mass which appears as energy.
Example of Nuclear Fission. When uranium-235 (U-235) atoms are bombarded with slow moving neutrons, the heavy uranium atom breakes up producing two medium weight atoms Barium (Ba) and krypton-94 with the emission of three neutrons and release of a great amount of energy.
The fission reaction can be represented in the form of following nuclear reaction :
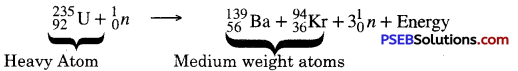
In the fission of uranium some mass of uranium is lost which results in the production of large amount of energy. The energy can be made use of for generating electricity.
Question 9.
What is Nuclear Fusion? Explain this phenomenon with the help of an example.
Or
Describe the process by which energy is released by the Sun.
Answer:
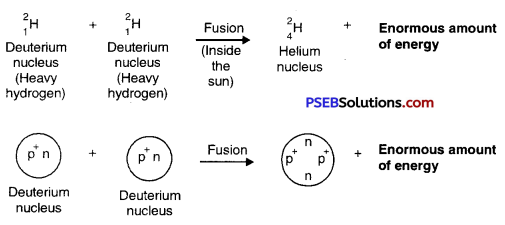
Nuclear Fusion: The process in which two nuclei of light atoms (hydrogen atom) combine to form a heavy and more stable atom (helium atom) with the liberation of a large amount of energy is called Nuclear Fusion.
This process is carried out by heating the light atoms to a very high temperature when some loss of mass occurs. This produces a large amount of energy. The energy produced in fusion reaction is much more than in a fission reaction.
Example of Nuclear Fusion. When Deuterium atoms (isotopes of heavy hydrogen atoms of mass number 2) are heated to an extremely high temperature, then two deuterium nuclei combine together to form a heavy nucleus of Helium and enormous energy is released. This nuclear reaction can be represented by the following equation :
- Energy from salinity gradient in seas: The concentration of salts in the waters of different seas is different. The difference in salt concentrations in the water of two different seas is called salinity gradien t. This difference in concentration of salt, where waters from two different seas meet, can be used to obtain energy in usable form.
- Energy from sea vegetation or bio-mass: Sea vegetation or biomass is also an indirect source of energy. For example, the vast sea weed plantation may provide an endless supply of methane fuel in future.
- Energy from the nuclear fusion of deuterium present in oceans: The oceans are also a source of deuterium (heavy hydrogen). Attempts are being made to produce energy by carrying out the controlled nuclear fusion of deuterium.
Question 10.
Write three points to distinguish between nuclear fission and nuclear fusion.
Answer:
Differences between Nuclear Fission and Nuclear Fusion.
| Nuclear Fission |
Nuclear Fusion |
| 1. In a fission reaction, a heavy nucleus breaks up to form two lighter nuclei. |
1. In a fusion reaction two light nuclei combine to form a heavy nucleus. |
| 2. Nuclear fission is a chain reaction. |
2. Nuclear fusion is not a chain reaction. |
| 3. Fission reactions are carried out by bombarding the heavy nuclei with neutrons. |
3. Fusion reactions are carried out by heating the light atoms to an extremely high temperature. |
| 4. Nuclear fission reactions can be controlled to generate electricity. |
4. Nuclear fusion reactions have not been controlled so far. |
| 5. Nuclear fission produces a large amount of energy. |
5. The energy produced in a nuclear fusion reaction is much more than that produced during fission. |
Short Answer Type Questions
Question 1.
What are fossils?
Answer:
Fossils: The hard parts of animals and plants or their ancient imprints on rocks which are found during excavation of rocks, are called fossils. As for example bones of animals, their skeletons, their foot prints are all fossils. This helps in the study of evolution and growth of organisms.
Question 2.
What are fossil fuels? What will happen if fossil fuels are used up at a fast rate? Give reasons for your answer.
Answer:
Fossil Fuels. These are the energy-rich compounds of carbon which were originally made by the plants with the help of solar energy. Fossil fuels like coal, petroleum and natural gas are formed under the earth over a long period of millions of years. So, if we continue to consume fossil fuels at this rapid rate, then all the fossil fuels will be exhausted one day. But the earth will not recreate fossil fuels so rapidly.

Question 3.
Why is L.P.G. considered an ideal fuel?
Answer:
L.P.G. an Ideal Fuel. Because of the following qualities, L.P.G. is considered as an ideal fuel :
- L.P.G. has high calorific value.
- L.P.G. has low ignition temperature.
- L.P.G. on combustion does not produce toxic gases and as such does not cause pollution.
- L.P.G. has balanced rate of combustion.
- L.P.G. contains much less incombustible substance.
- The combustion of L.P.G. is complete. Therefore, it does not leave behind any residue.
Question 4.
State the advantages of the nuclear fusion reactions over nuclear fission reactions. Also state one disadvantage.
Answer:
Advantages of Nuclear Fusion over Nuclear Fission.
- The amount of energy liberated in a fusion reaction is much more than that in a fission reaction.
- The products of a fusion reaction are not radioactive. They are harmless and hence can be disposed off easily.
- On the other hand, the products and by-products of fission reactions are radioactive and hence harmful. So the problem of their disposal arises.
Disadvantage. It has not been possible to have a controlled fusion reaction so far.
Question 5.
How is hydroelectricity generated at hydroelectric power plant? Give its advantages and disadvantages.
Answer:
The basic principle of hydroelectric power is that the potential energy of the water stored in the dams is converted into kinetic energy by allowing the water to fall
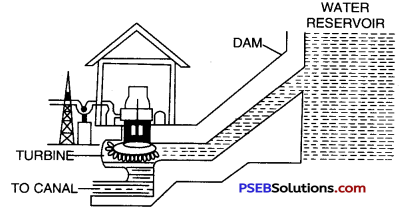
Outline of a hydroelectric power station.
from a height on turbines placed at the bottom of dam. The falling water makes the blades of turbines to rotate which in turn rotates the coil of generator placed in a magnetic field. This results in the production of electricity.
Limitations of Water Energy: The flowing water is not available in plenty everywhere to turn the turbines to produce electricity. Therefore, useful work can be obtained from water energy only at limited places where the flow of water is more.
Advantages and Disadvantages of Generating Hydroelectricity
Advantages:
- The use of water energy does not cause any pollution.
- It is an economical and renewable source of energy which will never get exhausted.
Disadvantages:
It causes a number of environmental problems. These are given below :
- The construction of dams on rivers results into a variety of ecological changes in the downstream area of the river. As a result a vast variety of flora and fauna (plants and animals) are destroyed and many people become homeless.
- The soil in the downstream area may become barren because there were no annual floods to deposit nutrient rich silt on the banks of the river. Therefore, there may be ecological imbalance.
Therefore, while constructing the high-rise dams to generate hydroelectricity, special care should be taken to study its impact on the environment and social life.
Question 6.
What is biogas? Give any four uses.
Answer:
Biogas: The mixture of Methane (CH4), Carbon dioxide (CO2) Hydrogen (H2), Nitrogen (N2) and Hydrogen Sulphide (H2S) obtained by degradation of plants and animals wastes in the presence of water due to anaerobic fermentation is called biogas. Its main constituent is Methane (50% – 70%).
Composition of Biogas
| Methane |
50% – 70% |
| Carbon dioxide |
30% – 40% |
| Hydrogen |
5% – 10% |
| Nitrogen |
1% – 2% |
| Hydrogen sulphide |
very small quantity |
Uses of Biogas:
- It is used as fuel for cooking food.
- It is used as a fuel to run engines.
- It is used for street-lighting.
Question 7.
Biogas is considered to be a boon to the farmers. Give reasons.
Answer:
Biogas is considered as a boon to the farmers because :
- They can produce clean fuel from wastes.
- They use spent up slurry as manure.
- They can generate electricity from biogas.
Question 8.
Give two reasons for using gobar (cow dung) in Biogas plant.
Answer:
Use of Gobar in Biogas Plant. The use of cow dung in biogas plants to prepare biogas is preferred because of the following reasons :
- When cow dung is used in biogas plant, the residue left after taking gas from the plant is still rich in nutrients and is used as manure.
- Biogas burns without producing any smoke, so it is a clean fuel and does not cause pollution.
Question 9.
Write the general principle involved in generating nuclear energy. Name one fuel used in a nuclear reactor.
Answer:
- Nuclear energy is generated by a process called nuclear fission.
- In this process nucleus of a heavy atom is bombarded with slow moving neutrons to split it and release a large amount of energy.
- Nuclear Fuel: Uranium, plutonium, thorium are used as nuclear fuels.
Question 10.
What is natural gas? What are its advantages over other fuels?
Answer:
Natural gas: It is a fossil fuel. It consists mainly of methane and small quantities of ethane and propane. Thus, methane is the principal constituent of natural gas (upto 97%). It occurs deep under the earth either alone or along with oil above of the petroleum deposits.
Question 11.
Which way of using cow-dung as fuel for domestic use is better : use of cow-dung cakes or use of cow-dung in a bio-gas plant? Give three reasons in support of your answer.
Answer:
When cow-dung cakes burn, they produces a lot of smoke, which causes air pollution. However, the use of cow-dung in biogas plant is better because of the following reasons :
- bio-gas burns without smoke.
- bio-gas produces a large amount of heat.
- the residue (slurry) left in the plant is rich in nitrogenous and phosphorus compounds and can be used as a useful manure.
Question 12.
Why burning firewood in traditional chulhas is not considered advantageous?
Answer:
Air pollution produced in atmosphere due to smoke in the absence of chimneys and so this is wastage of fuel as efficiency is low and calorific value is not so good. Carbon mono oxide produced due to partial burning of fuel is very much poisonous.
Question 13.
Expand LPG. What are its uses?
Answer:
LPG (Liquefied Petroleum Gas). The petroleum gas liquefied under pressure is called liquefied petroleum gas (LPG). It consists of mainly butane and small amounts of propane and ethane. This is a domestic gas which we ordinarily use.
Uses of LPG:
- It has a high calorific value.
- It does not produce any poisonous gases on burning.
- It is easy to handle and convenient to use.
- It does not cause pollution.
Question 14.
Explain why :
(a) Solar cookers are covered with glass plate.
Answer:
To attain the high temperature the solar cookers are covered with glass plate. This plate focuses the sun rays to a point and temperature inside the box is increased.
(b) The solar cooker is painted black from inside.
Answer:
The black surface of the solar cooker absorbs more heat as compared to white surface.
Question 15.
Write any two limitations of solar heating devices.
Answer:
Limitations of Solar Heating Devices:
- It saves fuel.
- The nutrients of food do not get destroyed during cooking.
- If does not produce pollution.
- Its maintenance cost is low.
- It is easy to handle and there is no danger of any mishap.
Question 16.
Why is it not possible to make use of solar cells to meet all our energy needs? State at least three reasons to support your answer.
Answer:
Solar cells do not meet all our energy needs due to :
- High cost of installation.
- Limited availability of special grade silicon to make solar cells.
- Unavailability of efficient system to store electrical energy generated by solar panels.
Question 17.
Write four areas where solar cell is used as source of energy?
Answer:
Uses of Solar Cells:
- It is used for generating electricity for operating on board artificial sattelites and spaceprobes.
- Solar cells are used to provide electricity to “light houses” situated in the sea and off shore oil drilling platform.
- Solar cells are used for operating electronic watches and calculators.
- Solar cells are used for street lighting, for operating water pumps and working of television in remote areas where electricity is not available.

Question 18.
Hydrogen gas has high calorific value but it is not used as a domestic fuel. Why?
Answer:
Though hydrogen has very high calorific value, yet it is not used as a domestic fuel because of the following reasons :
- It is highly combustible gas and its combustion cannot be controlled.
- Its transportation is difficult.
- Its cost of production is high.
Question 19.
What is sind mill? Write its two advantages.
Answer:
Windmill: A wind-mill is a machine which works with the energy of wind and converts kinetic energy of wind into mechanical energy or electricity. It consists of large fan like blades to catch the wind. When the wind strikes against these blades, the blades start rotating. This motion can then be passed on to connected crankshaft and is used to draw water from wells and to run flour mills for grinding grains.
Advantages of Wind Mill.
- Wind mill is used for drawing water from tube wells.
- Wind Mill for Grinding Grains. Wind mill is used to grind grains by suitable arrangement of toothed wheel and shaft.
Question 20.
On what basis would you classify energy sources as :
(а) renewable and non-renewable?
(b) exhaustible and inexhaustible?
Are the options given in (a) and (6) same?
Answer:
- Sources of energy that will get depleted some day are said to be exhaustible sources or non-renewable sources of energy.
- Sources from which we can be assured of constant supply of energy at a particular rate i.e., the sources which can be regenerated are called renewable sources of energy.
- Inexhaustive or renewable energy is one for which the depletion of the reservoir because of extraction of useable energy is practically negligible.
- We are getting energy from sun for last five billion years and we go on getting it for another five billion years, therefore we can say solar enegy is inexhaustible or renewable.
- Coal reserve with us are for about 200 years whereas it will take millions year for it to form again, hence coal may be said to be exhaustible or non-renewable source of energy.
- Thus inexhaustible is renewable and exhaustible is non-renewable.
Question 21.
What are fossil fuels? How are they formed? Why are they non¬renewable sources of energy?
Answer:
Fossil fuels. Fossil fuels are the energy rich compounds of carbon which are formed in many-many years from the remains of dead plants and animals in the absence of oxygen under the combined effect of pressure, temperature and bacteria.
Examples. Coal, petroleum and natural gas.
Formation of Fossil fuels. The plants and animals which died millions of years ago got buried deep under the earth away from reach of oxygen. The cover of sediments prevented their oxidation and they got squeezed due to the weight of sediments and got converted into water and volatile materials. Thus in the absence of oxygen and under the combined effect of high pressure, high temperature and bacteria. The remains of plants and animals were converted into fossil fuels. The fossils of smaller plants and animals got converted into oil and gas while the fossils of large plants got converted into coal.
Fossil fuel is non-renewable source of energy since it takes millions of years to form these fuels. It should be used only when it is needed.
Question 22.
What is greenhouse effect? Explain in detail.
Answer:
Greenhouse Effect. Carbon dioxide content of the air is increasing due to deforestation and combustion in industries, automobiles and planes, and is likely to become double by 2020. This increase is affecting the atmospheric composition and balance of gases, which are among the factors that control earth’s climate. Increase of carbon dioxide causes rise in atmospheric temperature, producing what is called the Greenhouse Effect. A rise of global temperature by more than 2 or 3 degrees may melt glaciers and polar ice. This will cause rise in ocean level and consequent flooding of coastal towns and submersible of islands. Rainfall pattern may also change, affecting agricultural output.
Question 23.
Differentiate between renewable and non-renewable sources of energy.
Answer:
Non-renewable sources of energy. Wood, fossil fuels like coal petroleum, natural gas, alcohol etc; Also called conventional sources of energy. Fossil fuels if consumed at the present speed are not going to last beyond 2050 AD. It takes millions of years to form fossil fuel. Such sources which cannot be formed again are called non-renewable sources of energy. Consumption of non-renewable sources cause high concentration of harmful gases. This has also led to depletion of ozone layer and global warming. Pollution caused by vehicles cause huge health hazards.
Renewable sources of energy. These sources are called non-conventional or renewable sources of energy. The sun, hydel (water) and wind energies can never get exhausted. All the renewable sources of energy are non-polluting and absolutely safe.
Geothermal energy derived from hot rocks, magma and hot water natural geyser, ocean thermal energy derived from waves and tidal waves are absolutely safe. Fuel cells use hydrogen and oxygen to produce electricity. Nuclear energy is the other source of non-conventional energy but it is very hazardous to harness.
Question 24.
“Oceans are vast store of energy”. Justify this statement.
Or
What is ocean thermal energy (OTE)?
Answer:
Oceans and seas are sources of large amount of energy. The following are factors responsible for energy production.
- Kinetic energy of sea waves can produce electricity.
- Tides and ebbs caused by moon and sun attraction can rotate turbines to produce electricity.
- Sea vegetation can be used as fuel.
Question 25.
What are solar reflectors and solar concentrators?
Answer:
Solar Reflectors. Those devices in which the sun rays are reflected by plane mirrors and let rays fall on containers which are black in colour. These containers absorb rays due to their black surface and get heated up, are called solar reflectors.
Solar Concentrators. When high temperature is needed, the sun rays are concentrated by parabolic mirror or converging lens at a point.
Main Components of a Solar cooker
- Blackened surface.
- Ordinary glass sheet.
- Suitable arrangement of mirrors.
- Insulated box.
Very Short Answer Type Questions
Question 1.
What are constituents of coal gas?
Answer:
It is a mixture of 55% hydrogen, 30% of methane, 10% of carbon monoxide and 5% of ethylene.
Question 2.
Write two examples of fossil fuels.
Answer:
Fossil fuels are those which are present inside earth’s crust as remains of animals and plants. Coal, petroleum, natural gas etc., form fossil fuels.
Question 3.
What is the range of temperature which can be attained in a box type solar cooker in two to three hours exposure to sun?
Answer:
100°C to 140°C.
Question 4.
Name the device which directly converts solar energy into electrical energy.
Answer:
Solar cell.
Question 5.
What is biogas?
Answer:
Biogas. It is a mixture of gases such as methane, carbon dioxide, hydrogen and hydrogen sulphide produced by the decay of biomass in the absence of air.
Question 6.
Name the main constituent of biogas.
Answer:
The main constituent of biogas is methane.
Question 7.
Name two main components of biogas.
Answer:
Methane (CH4) and hydrogen.

Question 8.
List two nutrients in which the slurry left behind in the digester is rich?
Answer:
It is rich in nitrogenous and phosphorus compounds.
Question 9.
What type of energy is possessed by huge waves near the seashore?
Answer:
Kinetic energy.
Question 10.
What is tidal energy?
Answer:
The energy possessed by the rising and falling water in tides.
Question 11.
Define energy.
Answer:
Energy. The ability to do work is energy.
Question 12.
Define kinetic energy.
Answer:
Kinetic energy. The energy possessed by a body due to its motion is called kinetic energy.
Question 13.
Name three fossil fuels.
Answer:
Coal, Petroleum and Natural gas.
Question 14.
Define Solar Energy.
Answer:
Solar Energy. The energy produced by sun is called solar energy.
Question 15.
What is calorific value of a fuel?
Answer:
Calorific value. The heat produced by full burning of unit mass of fuel is called calorific value of the fuel.
Question 16.
From which substances is biogas obtained?
Answer:
It is obtained from plants and animals waste, cattle dung, domestic waste, wastes from poultry.
Question 17.
Name the constituents of Biogas.
Answer:
CH4, CO2 and H2S. The percentage of methane is 65%.
Question 18.
Name any two materials used for making of solar cells.
Answer:
Selenium, gallium, silicon etc.
Question 19.
Define wind energy.
Answer:
Wind energy. The energy possessed by moving air (wind) is called wind energy. Wind possesses kinetic energy due to its motion.
Question 20.
What is biomass? Write its uses.
Answer:
Biomass is the decomposed mass of animals and plants. It is the total matter present in the bodies of plants and animals.
Uses:
- It is used as a biofuel.
- These give energy by burning.
Question 21.
Which type of mirror is most suitable in the device shown in figure below?
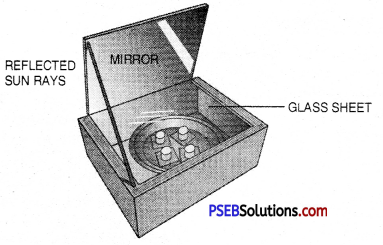
Answer:
Plane Mirror.

Question 22.
Name the device shown in the given diagram. What is being prepared in it?
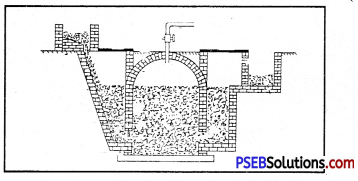
Answer:
The device shown in the figure is Fixed Dome type Biogas Plant. In this plant Biogas is being Prepared.
Question 23.
Label 1 and 2 in the given figure.
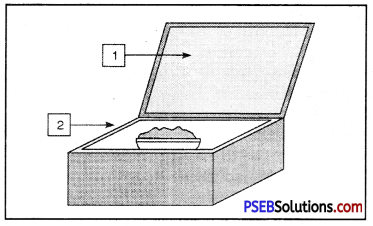
Answer:
1 – Plane mirror
2 – Wooden Box
Multiple Choice Questions
Question 1.
Non-renewable source ________
(A) Solar energy
(B) Wind energy
(C) Natural gas
(D) Water.
Answer:
(C) Natural gas
Question 2.
________ is used in Hydro-electric power station.
(A) Wind energy
(B) Energy of running water
(C) Energy produced from burning coal
(D) All of these.
Answer:
(B) Energy of running water
Question 3.
________ is used in thermal power station.
(A) Wind energy
(B) Energy of flowing water
(C) Energy produced from burning fossil fuel
(D) All of these.
Answer:
(C) Energy produced from burning fossil fuel
Question 4.
The temperature range of Box Type solar cooker kept in the sun for 2-3 hours will be ________
(A) 60° C – 80° C
(B) 80° C – 100° C
(C) 100° C – 140° C
(D) 140° – 180° C.
Answer:
(C) 100° C – 140° C
Question 5.
________ converts solar energy into electric energy
(A) Solar water heater
(B) Solar cell panel
(C) Solar furnace
(D) Solar cooker.
Answer:
(B) Solar cell panel
Question 6.
________ is used in making solar cell
(A) Carbon
(B) Silicon
(C) Sodium
(D) Cobalt.
Answer:
(B) Silicon

Question 7.
The device that converting energy into mechanical energy is called ________
(A) Solar cooker
(B) Solar cell
(C) Engine
(D) All the above.
Answer:
(C) Engine
Question 8.
Which of the following is not a source of Bio-mass energy?
(A) Wood
(B) Gobar gas
(C) Coal
(D) Nuclear energy.
Answer:
(D) Nuclear energy.
Question 9.
Which one of the following is not a solid fuel?
(A) Coal
(B) Wood
(C) Coke
(D) Kerosene.
Answer:
(D) Kerosene
Question 10.
Which one is a non-renewable / source of energy?
(A) Wind
(B) Sun
(C) Fossil fuel
(D) Water.
Answer:
(C) Fossil fuel
Question 11.
The main source of energy is ________
(A) Sun
(B) Water
(C) Uranium
(D) Fossil fuel.
Answer:
(A) Sun
Question 12.
Slurry obtained from Biogas Plant contains maximum quantity of ________
(A) Sulphur, Phosphorus
(B) Carbon, Sodium
(C) Nitrogen, Oxygen
(D) Phosphorus, Nitrogen.
Answer:
(D) Phosphorus, Nitrogen
Question 13.
Which country is called the country of Winds?
(A) India
(B) Italy
(C) France
(D) Denmark.
Answer:
(D) Denmark
Question 14.
The combination of solar cells is called ________
(A) Solar Panel
(B) Solar Band
(C) Solar Circle
(D) Solar Plate.
Answer:
(A) Solar Panel
Question 15.
The element used in manu¬facturing solar cells is ________
(A) Copper
(B) Tungsten
(C) Sulphur
(D) Silicon.
Answer:
(D) Silicon
Question 16.
The vast source of energy on the earth is ________
(A) Wood
(B) Coal
(C) Sun
(D) Moon.
Answer:
(C) Sun
Question 17.
Natural gas is mostly :
(A) Methane
(B) Ethane
(C) Propane
(D) Pentane.
Answer:
(D) Pentane.
Question 18.
Solar water heater cannot be used to get hot water on :
(A) Sunny day
(B) Cloudy day
(C) Hot day
(D) Windy day.
Answer:
(A) Sunny day.
Question 19.
Which one of the following energies are freely available?
(A) Bio gas
(B) Sunlight
(C) Water gas
(D) Hydrogen.
Answer:
(B) Sunlight.
Question 20.
Which of the following is needed for formation of biogas from cow dung?
(A) Water
(B) Oxygen
(C) Carbon dioxide
(D) Hydrogen.
Answer:
(B) Oxygen
Fill in the blanks:
Question 1.
Non-renewable sources of energy must be used ________ as possible.
Answer:
rarely.
Question 2.
Sun produces huge amount of energy due to nuclear ________
Answer:
fusion.
Question 3.
The slurry left behind in a biogas plant is a good ________
Answer:
manure.

Question 4.
The fuels obtained from under the earth are called ________ fuels.
Answer:
fossil.
Question 5.
A black surface absorbs ________ heat as compared to white surface.
Answer:
more.
![]()
![]()
![]()
![]()
![]()
![]()