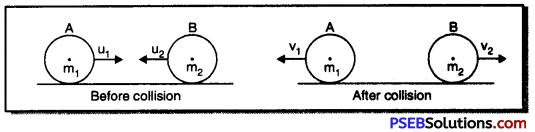
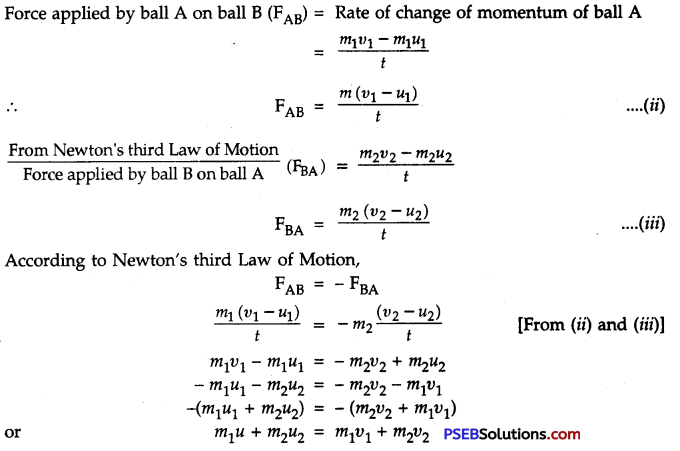
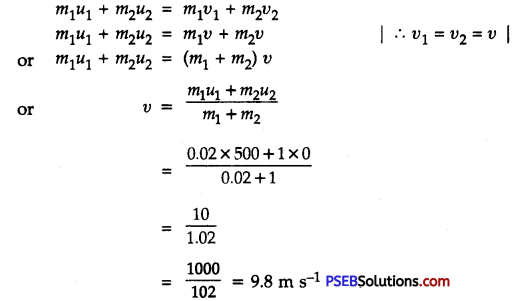
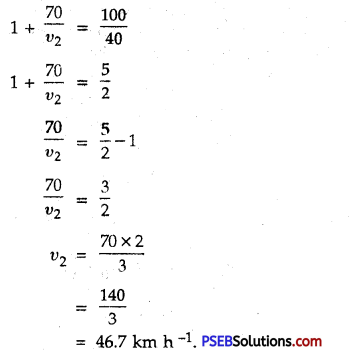
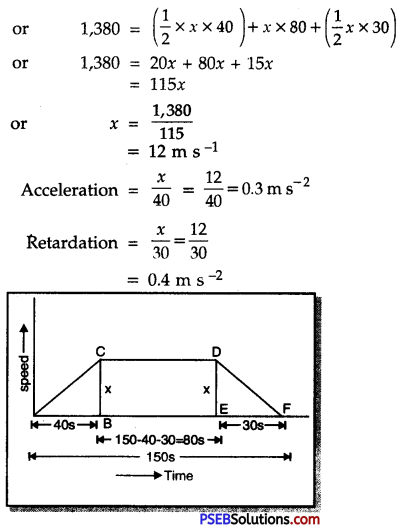
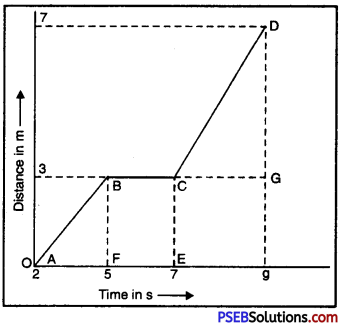
Punjab State Board PSEB 9th Class Science Important Questions Chapter 14 Natural Resources Important Questions and Answers.
PSEB 9th Class Science Important Questions Chapter 14 Natural Resources
Long Answer Type Questions:
Question 1.
Write a note on formation of soil.
Answer:
Formation of Soil: The formation of soil depends on the parent rock material, the climate and topography of the area, the organisms present in the soil and the time over which the soil has been developing. Over long periods of time, thousands and millions of years, the rocks near the surface of the Earth are broken down by various physical, chemical and some biological processes. The end product of this breaking down is the fine particles of soil.
Processes for soil formation:
1. The Sun: The sun heats up rocks during the day so that they expand. At night, the rocks cool down and contract. The unequal expansion and contraction in different parts of the rock results in the formation of cracks and ultimately rocks break up into smaller pieces.
2. Water: Water helps in the formation of soil in two ways:
- Water could get into the cracks in the rocks formed due to uneven heating by the sun. If this water freezes, it will widen the cracks.
- Fast flowing water carries big and small particles of rock downstream, causing breakdown of rock particles into smaller, finer particles through their abrasive effects.
3. Wind: Strong winds also break down rocks. They also carry sand from one place to the other like the water does.
4. Living organisms: They also influence the formation of soil. While lichens grow on surface of rocks, they release certain substances that cause the rock surface to powder down and form a thin layer of soil. Likewise, small plants like moss and roots of big trees also break the rocks.
![]()
Question 2.
What are the effects of use of fertilizers and pesticides for long period on soil fertility? What are the causes of soil erosion?
Answer:
Effects of excessive use of fertilizers and pesticides:
- Use of these substances over a long period of time can destroy the soil structure by killing the soil micro-organisms that recycle nutrients in the soil.
- They also kill the earthworms which are instrumental in making the rich humus.
- Fertile soil can quickly be turned barren if sustainable practices are not followed.
- Major cause of soil pollution is removal of useful components from the soil and addition of other substances, which adversely affects the fertility of the soil and kills the diversity of organisms living in it, is called soil pollution.
Causes of Soil Erosion:
- Wind causes soil erosion by carrying away the top loose soil particles.
- Rain causes soil erosion on unprotected topsoil by washing it down.
- mproper farming or tilling and leaving the field fallow for long time causes soil erosion.
- Frequent flooding of rivers causes soil erosion by removing the fertile top soil of the fields near the river banks.
- Deforestation also leads to soil erosion.
Question 3.
Why is soil as resource important for mankind? Mention the constituents of soil.
Answer:
Soil is a rich source of minerals and humus. It is important for growing crops. Soil water is used by plants for various functions.
Soil provides support to crops, grassland and forests thus it is an important natural resource.
Components of Soil. Soil is a mixture, it contains:
- Small particles of rocks.
- Bits of decayed living organisms which is called humus.
- Soil also contains various forms of microscopic life.
- It contains nutrients and availability of which depends on the rocks from which it was formed.
- Soil water – 25% – 35%
- Soil air – 15-25 %
Question 4.
Define soil fertility. How can it be maintained?
Answer:
Soil fertility. It is the ability of soil to provide minerals, water and other nutrients to the plants.
Conservation of Soil fertility:
- Adding of manure to the soil.
- Rotation of crops.
- Keeping the land as such without growing any crop.
- Addition of fertilizers.
Artificial methods to maintain soil fertility:
- Nitrogenous and other fertilizers are added.
- For natural restoration of nutrients, soil is kept uncultivated for certain period.
Role of humus:
- Humus increases the soil fertility.
- Humus has high retaining capacity for water.
- It makes the soil porous and allows water and air to penetrate deep.
![]()
Question 5.
What is air pollution? Write the main sources and preventive measures.
Answer:
Air Pollution. Air pollution refers to the release into the atmosphere of materials that are harmful to man, other animals, plants and buildings or other objects.
Sources of Air pollution:
The major sources of air pollution are fossil fuels (coal and petroleum) and industries. Human Sources. Many activities done by man are the main sources of air pollution. These activities can be divided into following categories.
- Combustion activities.
- Industrial activities.
- Agricultural works.
- Use of solvents.
- Activities concerned with atomic energy.
Preventive measures for air pollution:
To prevent and control air pollution two types of measures can be adopted.
1. Instead of releasing poisonous gases containing various pollutants into the atmosphere they could be destroyed or used by some other measures.
2. Converting harmful pollutants to harmless products and then releasing them into
the atmosphere.
Control measures for minimizing air pollution:
1. Simple combustible solid wastes should be burnt in incinerators.
2. Automobiles must be either made to eliminate the use of gasoline and diesel oil or complete combustion is obtained in the engine so that harmful products are omitted.
Question 6.
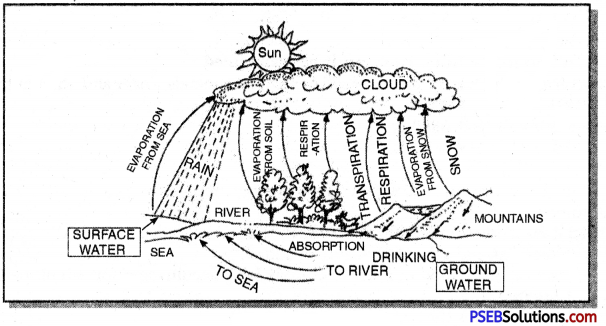
Write a few properties of water. Sketch water Cycle.
Answer:
Water is a liquid at room temperature. It is densiest (heaviest) at about 4°C. The dense water sinks and the lighter frozen water (ice) floats, ice also insulates the water below. This enables the aquatic life to survive under the ice in cold weather.
Question 7.
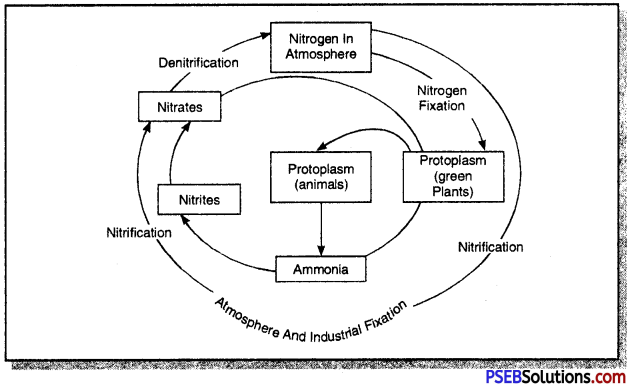
What is nitrogen cycle? Make a simple line sketch to show nitrogen cycle in the biosphere.
Answer:
The nitrogen cycle in the biosphere is regarded as a perfect cycle because the cycling process keeps the overall amount of nitrogen constant in the atmosphere and water bodies. The use of chemical nitrogeneous fertilizers like NPK and urea also help in the maintenance of soil nutrients and nitrogen cycle.
However, some of the nitrogen compounds present in soil get trapped within sedimentary rocks and therefore, they are not available to nitrogen cycle for circulation in the biosphere. However, this loss is compensated by volcanic eruptions and erosions and sedimentary rocks. Both these processes release nitrogen.
Micro-organisms involved in Nitrogen Cycle:
As already learnt, micro-organisms play a very important role in nitrogen cycle in nature. Different organisms are involved in different processes of nitrogen cycle. The main micro-organisms involved in nitrogen cycle are listed below:
Activity:
- Nitrogen fixation
- Ammonification
- Ammonia to nitrites
- Nitrification (Nitrites to nitrates)
- Denitrification (Nitrates to free Nitrogen)
Organisms:
- Rhizobium, blue-green algae
- Decay bacteria, fungi
- Nitrosomonas
- Nitrobacter
- Pseudomonas
The nitrogen cycle in the biosphere involves the following steps:
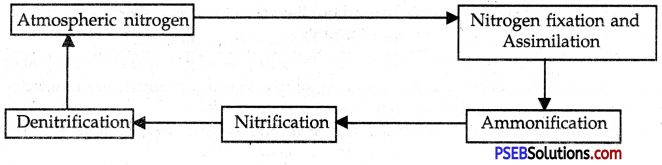
![]()
Question 8.
Briefly explain oxygen cycle.
Answer:
Oxygen cycle: Oxygen is very abundant element on earth. It is found in the elemental form in the atmosphere to the extent of 21%. It also occurs extensively in the combined form in the earth’s crust as well as also in the air in the form of carbon dioxide. In the crust, it is found as the oxides of most metals and silicon, and also as carbonate, sulphate, nitrate and other minerals. It is also an essential component of most biological molecules like carbohydrates, proteins, nucleic acids and fats (or lipids).
But when we talk of the oxygen cycle, we are mainly referring to the cycle that maintains the levels of oxygen in the atmosphere. Oxygen from the atmosphere is used up in three processes, namely combustion, respiration and in the formation of oxides of nitrogen. Oxygen is returned to the atmosphere in only one major process, that is, photosynthesis. And this forms the broad outline of the oxygen cycle in nature.
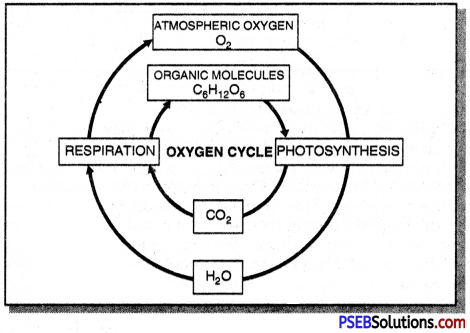
Question 9.
Briefly explain carbon cycle in nature.
Answer:
The Carbon Cycle. Carbon is found in various forms on the earth. It occurs in the elemental form as diamond and graphite. In abiotic environment, carbon is present in the following forms:
- as carbon dioxide in the atmosphere.
- as carbonate and hydrogen-carbonate salts in various minerals.
- as dissolved carbonic acid and bicarbonates in water.
- as fossil fuels like coal, petroleum and natural gas.
- Plants utilise the atmospheric carbon dioxide in photosynthesis to produce carbohydrates, which are taken by herbivores and then pass through small and large carnivores.
Forms of Carbon: Carbon is found in various forms on the earth.
- It occurs in the elemental form as diamonds and graphite.
- In the combined state, it is found as carbon dioxide in the atmosphere, as carbonate and hydrogen-carbonate salts in various minerals.
- All life forms are based on carbon-containing molecules like proteins, carbohydrates, fats, nucleic acids and vitamins.
- Both plants and animals release carbon dioxide to the atmosphere as a product of respiration.
- By decomposition of organic wastes and dead bodies by decomposers.
- By burning of fossil fuels, like coal, and petroleum.
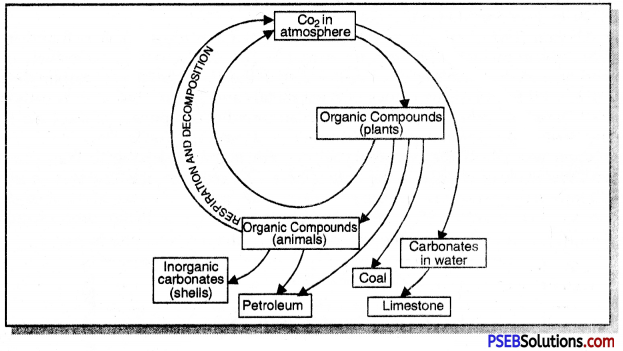
Question 10.
What are the causes of ozone depletion?
Answer:
Ozone depletion: Recently it was discovered that ozone layer was getting depleted. Various man-made compounds like CFCs (carbon compounds having both fluorine and chlorine which are very stable and not degraded by any biological process) were found to persist in the atmosphere.
Once they reached the ozone layer, they would react with the ozone molecules. This resulted in a reduction of the ozone layer and recently they have discovered a hole in the ozone layer above the Antarctica. It is difficult to imagine the consequences for life on earth if the ozone layer dwindles further, but many people think that it would be better not to take chances. Measures should be taken towards stopping all further damage to the ozone layer.
![]()
Question 11.
Write a note on freshwater resources.
Answer:
Fresh Water Resources:
Fresh water resources range from ponds to lakes and large rivers. It has the following characteristics :
(a) Freshwater is exhaustible, however, it is being made available again by oceans through hydrological cycle.
(b) Out of this three per cent, 77.2 per cent is stored in glaciers and ice caps. And 22.4 per cent is groundwater and soil moisture. Remaining 0.36 per cent is found in lakes, rivers, streams and swamps etc. Out of the total water evaporated from oceans 90 per cent falls on the oceans and remaining 10 per cent falls on the land. This water is utilised by various terrestrial ecosystems.
(c) Freshwater is essential for life on earth as well as for survival of human race.
(d) The total water in hydrosphere is 1.4 billion cubic kilometres (Km3). Total ocean
water is 97%. The ocean water cannot be consumed by human beings. Remaining three percent (freshwater) is available for human consumption. The water resources in India have an average run off in river system of 1,869 km2 and 432 km3 groundwater.
Short Answer Type Questions:
Question 1.
What is atmosphere? Name its different layers.
Answer:
Atmosphere: Gaseous envelope surrounding the earth is called atmosphere. Several concentric layers can be identified in vertical profile of atmosphere. Density, temperature and composition differ in these layers. Near the earth’s surface, density is highest and with increase in latitude density decreases. Starting from earth’s surface five concentric layers can be distinguished:
- Troposphere
- Stratosphere
- Mesosphere
- Thermosphere
- Ionosphere.
Exosphere which forms the outer fringe of atmosphere is highly rarefied and gradually get mixed with other space.
Composition of dry atmosphere:
| Components | Volume |
| Nitrogen (N2) | 78% |
| Oxygen (O2) | 21% |
| Carbon dioxide (CO2) | 0.03% |
| Argon | 0.93% |
| Helium, Neon, Ozone, ammonia | 0.04% |
Helium, Neon and Argon are noble gases.
Question 2.
What is the role of atmosphere in climate control?
Answer:
Role of atmosphere in climate control. Atmosphere covers the earth like a blanket. Air is a bad conductor of heat. The atmosphere keeps the average temperature of the earth fairly steady during the day and even during the course of the whole year. The atmosphere prevents the sudden increase in temperature during the daylight hours.
During the night, it slows down the escape of heat into outer space. Moon, which is about the same distance from the sun that the earth is. Despite that, on the surface of the moon, with no atmosphere, the temperature ranges from -190° C to 110° C.
![]()
Question 3.
What is ozone layer? Write its importance.
Answer:
Ozone layer: It is the protective layer. Ozone in stratosphere is responsible for protecting the earth from high energy ultraviolet radiation. It forms a life-saving screen as it checks the entry of lethal UV- rays. Ozone found in troposphere has warming effect. Ozone is one gas which is harmful as well as useful for human beings.
16th September, 1996 was celebrated as “International Day for the Preservation of Ozone layer”. It was aimed to generate awareness about the dangers of ozone depletion in the stratosphere and it was drawn up by UNEP.
Question 4.
What is meant by ozone shield? How the CFC’s and ozone-depleting substances effect ozone shield?
Answer:
Ozone shield: An equilibrium is established between generation and destruction of O3, leading to a steady-state concentration of ozone layer in the stratosphere between 20 and 26 km above the sea level. The thickness of the vertical column of stratospheric O3 layer, condensed to standard temperature and pressure, average 0.29 cm above the equator and may exceed 0.40 cm above the poles at the end of the winter season. This layer acts as the ozone shield protecting the earth biota from harmful effects of strong UV radiations.
CFC’s produce active chlorine (Cl with CIO radical) in the presence of UV radiations. These radicals catalytically destroy ozone converting it into oxygen. CH4 and N2O also cause ozone destruction.
Question 5.
Write a note on the air pollution caused due to combustion.
Answer:
The mobile combustion sources are the main sources of air pollution especially in the cities. They include the locomotives, automobiles and aircrafts.
The air pollutants from these are:
1. (i) Carbon monoxide (ii) oxides of nitrogen and (in) a mixture of hydrocarbons.
2. The petroleum used as fuel in these sources contains lead as an impurity in the form of tetraethyl lead Pb (C2H5)4, and tetramethyl lead Pb (CH3)4.
Question 6.
Discuss harmful effects of air pollution.
Answer:
Harmful Effects of Air Pollution
1. Air pollution affects the respiratory system causing breathing difficulties and diseases such as bronchitis, asthma, lung cancer, tuberculosis and pneumonia.
2. Burning of fossil fuels like coal and petroleum releases oxides of nitrogen and sulphur. Inhalation of these gases is dangerous. These gases also dissolve in rain to give rise to acid rain.
3. The combustion of fossil fuel also increases the amount of suspended particles in air. These suspended particles could be unbumt carbon particles or substances called hydrocarbons. The presence of high levels of all these pollutants, reduce visibility in cold weather where water also condenses out of air forming smog. Smog is an indication of air pollution.
4. Regular breathing in the polluted air increases allergies, cancer and heart diseases.
Question 7.
What is the role of biotic components in living organisms?
Answer:
The living or biotic components are plants and animals including us and non¬living or physical components are air, water, soil, light and temperature. All these components interact and effect each other, resulting in the establishment of a complex and complete balance in the environment. The environmental components like mountains, rivers, ponds, forests, minerals, coal and even petrol and other natural resources are of great importance to us.
CO2 is fixed in two ways:
1. Green plants convert CO2 into glucose in the presence of sunlight and chlorophyll pigments.
2. Many marine animals use carbonates dissolved in sea water to make their shells. Oxygen is required by eukaryotic and many prokaryotic cells. All the cells need oxygen to break down glucose molecules in order to release energy required to carry out this vital functions of life.
![]()
Question 8.
What are aerosols?
Answer:
Aerosols. Aerosols are certain chemicals released in the air with force in the form of mist or vapour. The important source of aerosols is the jet aeroplane emissions in the outer atmosphere. The aerosols contain fluorocarbons which deplete the ozone layer in the atmosphere.
Question 9.
What are acid rains?
Answer:
Acid Rain. It is the rain which contains small amount of acid in it that is formed from the gases like sulphur dioxide and nitrogen oxides present in polluted air. It causes damage to living and non-living things.
Question 10.
What is smog?
Answer:
Smog. Smoke and fog when combined together forms smog in the presence of sunlight. Various unbumt hydrocarbons produced from the automobile combustion react with oxides of nitrogen to form ozone, peroxyacyl nitrates and aldehydes. They are called photochemical oxidants. Together with smoke and fog they constitute smog which has a harmful effect on humans repiratory and nervous system; it also harm the plants and rubber goods.
Question 11.
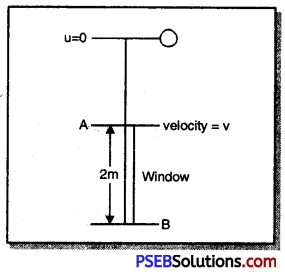
Explain the role of sun in soil formation.
Answer:
Role of sun in soil formation:
The sun heats up rocks dtiring the day as a result that they expand. At night, these rocks cool down and contract. Since all parts of the rock do not expand and contract at the same rate, this results in the formation of cracks and ultimately the huge rocks break up into smaller pieces and small pieces further break up into still smaller pieces and fine particles.
![]()
Question 12.
How does water take part in soil formation?
Answer:
Role of water in soil formation:
1. Water enters into cracks formed as a result of uneven heating by sun. As this water freezes, it causes the cracks in the rocks to widen.
2. Flowing water wears away hard rocks over long period of time. The fast flowing water often carries big and small particles of rock downstream. These pieces of rock rub against other rock pieces and the abrasion causes the rocks to wear down into smaller and still smaller pieces.
Question 13.
Discuss the role of wind and living organisms in soil formation.
Answer:
Role of wind in the soil formation. Strong wind rubs against rocks and wear them down. The wind also carries soil particles from one place to another.
Role of living organisms in soil formation:
Living organisms also influence the formation of soil. The lichen that we read about earlier, also grows on the surface of rocks. While growing, they release certain substances that cause the rock surface to powder down and form a thin layer of soil.
Other small plants like moss, are able to grow on this surface now and they cause the rock to break up further. The roots of big trees sometimes go into cracks in the rocks and as the roots grow bigger, the crack further becomes bigger causing the rocks to break down to form soil.
Question 14.
What is the role of atmosphere in movement of air which causes winds?
Answer:
The movement of air causes winds:
1. The atmosphere gets heated from the radiation that is reflected back or re-radiated by the land or water bodies. As a result of heating convection currents are set up in the air. Since land gets heated faster than water, the air over land gets heated faster than the air above water bodies.
2. In coastal regions, during the day, the air above the land gets heated faster and starts rising. So a region of low pressure is created and air over sea moves into this area of low pressure. The movement of air from one region to the other region causes winds.
3. During the day, the direction of wind would be from the sea to the land and at night, both land and sea starts to cool. Since water cools down slower than the land, the air above water would be warmer than the air above land, thus the direction of wind would be from the land to the sea.
![]()
Question 15.
How are rainfall patterns decided?
Answer:
- Rainfall patterns are decided by the prevailing wind patterns.
- In large parts of India, rains are mostly brought by the southwest or northeast monsoons.
- Depressions in the Bay of Bengal have caused rains in some areas, is the common comment during weather ireports.
Question16.
What is the role of atmosphere in causing rain?
Answer:
Role of atmosphere in causing rain:
- When water bodies are heated during the day, a large amount of water evaporates and goes into the air.
- The wind carries the water vapour to various places.
- The air gets heated and rises up carrying the water vapour with it.
- As this air rises, it expands and cools causing the water vapour in the air to condense in the form of tiny droplets.
- Once the water droplets are formed, they grow bigger by the ‘condensation’ of these water droplets.
- When the drops grow big and heavy, they fall down in the form of rain.
Question 17.
Comment “Water is one of the major resources which determine life on land”. List a few other factors also.
Answer:
The availability of water decides not only the number of individuals of each species that are able to survive in a particular area, but it also decides the diversity of life there. Of course, the availability of water is not the only factor that decides the sustainability of life in a region. Other factors like the temperature and nature of soil also matters. But water is one of the major resources which determine life on land.
Question 18.
What is water pollution?
Answer:
Water pollution: Addition of harmful materials to water is termed water pollution. The sources of inland water pollution are community wastewater (sewage) and wastes from industries and agricultural practices. Water pollutants include organic matter, pathogens, chemicals and minerals, solid particles, radioactive wastes and heat.
![]()
Question 19.
What are the sources of water pollution?
Answer:
Sources of Water Pollution:
- Agriculture substances such as fertilisers and pesticides are used to increase crop yield, and some amount of these chemicals is washed into the water bodies that pollutes the water.
- Sewage from homes and wastes from factories are dumped into rivers or lakes also cause water pollution.
- Hot and cold water discharged from industries make a change in temperature, which is harmful for aquatic organisms.
- All these affects the balance among various organisms that are found in water bodies.
Question 20.
What are the effects of water pollution?
Answer:
Effects of Water Pollution:
- Water pollutants reach the sea directly from the coastal cities and ships, and indirectly with river water from distant places. Oil spilled in tanker accidents is a major threat to ocean life.
- The substances like fertilisers and pesticides used in farming, mercury salts used by paper industries could be poisonous. There could also be disease-causing organisms, like the bacteria which causes cholera.
- Industrial or household waste reduces the dissolved oxygen in water bodies, thereby affecting the aquatic life.
- Aquatic organisms can stay alive in a certain range of temperature. Sudden change in temperature of water bodies is dangerous for aquatic organisms and affects their breeding.
Question 21.
Make a list of various diseases caused by polluted water.
Answer:
Diseases caused by polluted water
- Bacterial diseases. Cholera, Typhoid, Diarrhoea, Dysentery.
- Viral diseases. Jaundice, Polio etc.
- Protozonal diseases. Diseases associated with stomach and intestines eg. Amoebic dysentery, Giardiasis etc.
- Helminthic diseases. Infection of some intestinal parasites like Ascaris lumbricodies is through drinking water only.
- Guinea worm diseases is through Cyclops present in the drinking water. Through contaminated water they reach to another host i.e. man.
Question 22.
What is greenhouse effect? Show the % age of gases that cause greenhouse effect.
Answer:
Greenhouse effect. Earth’s temperature is maintained by reradiated infra-red radiations by CO2, CH4, O3, NO and NO2 and slightly by water vapours in atmosphere. These gases prevent heat from escaping to outer space, so are functionally comparable to glass panels of a greenhouse and are called greenhouse gases (GHGs) and the process is called greenhouse effect. The CO2 is added to atmosphere mainly by burning fossil fuels, volcanic activities and respiration.
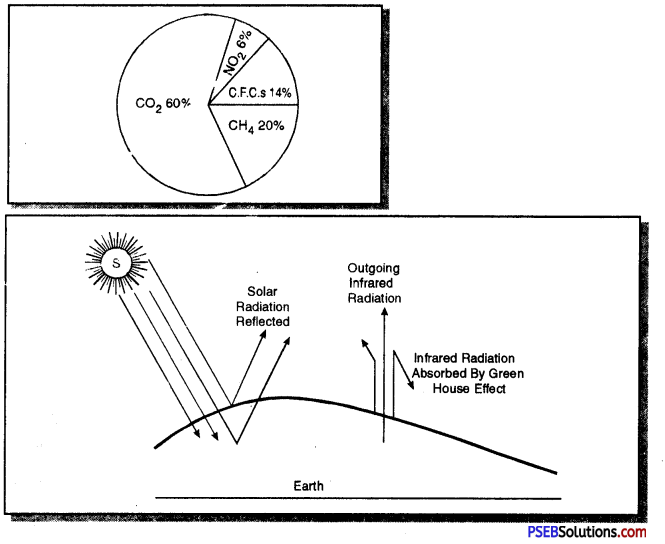
Question 23.
List Various Measures for Soil Conservation
Answer:
Various Measures for Soil Conservation:
- Stopping clear-cutting of forests and overgrazing checks soil erosion by streams and rivers.
- Intensive cropping helps in checking soil erosion. A field always under a crop is protected against erosion.
- Bunds around the fields contain rain water and check soil erosion besides washing away of minerals.
- Irrigation channels in the fields should be so designed as to carry water at a slow speed.
- Drainage canals to carry flood water will protect the fields against erosion.
![]()
Question 24.
How will you determine composition of soil?
Answer:
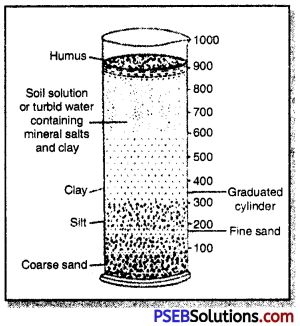
Determination of Soil Composition:
- Materials required: 150 cc of sifted soil, a measuring glass cylinder of 1 litre capacity, water, glass rod.
- Procedure: Take 150 cc of sifted soil in a glass cylinder. Pour about 750 cc of water over it. Stir the soil well with the help of a glass rod. Take out the rod. Allow the particles to settle. Observe after 30 minutes.
- Results: The bottom of the cylinder has a layer of coarse sand. A layer of fine sand lies above it. Then there is a layer of silt. Clay lies above silt.
Turbid water occurs above the clay. It contains clay as well as mineral salts. Humus or organic matter floats over the top of turbid water.
Question 25.
Make an outline sketch of nitrogen cycle.
Answer:
Nitrogen Cycle
Very Short Answer Type Questions:
Question 1.
Life exists on which planet?
Answer:
Earth.
![]()
Question 2.
What are the materials required for life?
Answer:
Environment, Heat, Light, Water and food.
Question 3.
What is environment?
Answer:
Environment. The earth and everything which affects the living organisms constitute its environment.
Question 4.
Basic requirment of life are obtained from which sources.
Answer:
Energy from sun and resources present on earth.
Question 5.
What is atmosphere?
Answer:
Atmosphere: It is the multilayered gaseous envelope of air that covers the whole of the planet earth like a blanket.
Question 6.
How much surface of earth is covered by water?
Answer:
About 75 percent.
![]()
Question 7.
How atmosphere covers the earth?
Answer:
Atmosphere covers the earth as a blanket.
Question 8.
What is biosphere?
Answer:
It is the life-supporting zone of earth where the atmosphere, the hydrosphere and the lithosphere interact and make life possible.
Question 9.
What are biotic components of biosphere?
Answer:
All living organisms.
Question 10.
List the abiotic components of bioshphere?
Answer:
Air, water and soil.
Question 11.
Name the gaseous components of atmosphere.
Answer:
Nitrogen, Oxygen, CO2 and Water vapour.
Question 12.
Name the gases present on Venus and Mars planet.
Answer:
95 to 97% CO2.
Question 13.
What is respiration?
Answer:
A process in which O2 is used and CO2 is liberated.
Question 14.
How is CO2 used so that balance is maintained?
Answer:
1. CO2 is used during photosynthesis and carbohydrates are formed.
2. Used as carbonates by marine molluscs.
![]()
Question 16.
How is temperature regulated on earth?
Answer:
Atmosphere regulates the temperature on earth.
Question 17.
What is the minimum and maximum temperature on moon?
Answer:
Minimum temperature = – 190°C
Maximum temperature = 110°C.
Question 18.
What causes wind?
Answer:
Movement of air caused by uneven heating of the atmosphere in different regions of earth.
Question 19.
How are clouds formed?
Answer:
Clouds are formed by condensation of water droplets in the air.
Question 20.
Which wind gets hot : Water to earth surface or from surface of earth to upward.
Answer:
Land (surface of earth) to upward.
Question 21.
During night what is the direction of movement of air?
Answer:
From surface of earth (land) to the sea.
![]()
Question 22.
What is the cause of movement of wind?
Answer:
Interaction of atmospheric components.
Question 23.
List two factors which affect wind.
Answer:
1. Rotation of earth
2. Presence of mountain heights.
Question 24.
What is deforestation?
Answer:
Cutting of trees on large scale is called deforestation.
Question 25.
What is the effect of deforestation?
Answer:
Deterioration of atmosphere.
Question 26.
Is air a good or bad conductor of heat?
Answer:
Air is a bad conductor of heat.
Question 27.
What is the cause of rain on Indian Land.
Answer:
Rain in India is due to monsoon from south-west or east-west direction.
Question 28.
What is smog?
Answer:
Smoke mixed with fog is called smog.
![]()
Question 29.
What does the smog indicate?
Answer:
It indicates pollution of air.
Question 30.
Where is the purest form of water available.
Answer:
Snow/Ice caps.
Question 31.
Write one importance of water for living organisms.
Answer:
All cellular processes take place in water medium in living organisms.
Question 32.
Write one cause of pollution of water in town.
Answer:
Sewage.
Question 33.
What is soil?
Answer:
The top weathered part of earth’s surface is called soil.
Question 34.
What is the role of sun in the formation of soil?
Answer:
Heating of rocks causes cracking and ultimately breaking up into smaller pieces.
![]()
Question 35.
What is the role of wind in soil formation?
Answer:
Wind causes erosion of rocks.
Question 36.
What is soil erosion?
Answer:
Removal of useful components from the soil which affect the fertility of soil is called soil erosion.
Question 37.
What is the importance of soil?
Answer:
Soil supports terrestrial plants and animals and it decides the diversity of life in an area.
Question 38.
How is soil formed?
Answer:
Soil is formed by weathering of rocks.
Question 39.
What are three kinds of water sources?
Answer:
Rainwater, Groundwater and Subsoil water.
![]()
Question 40.
Write one advantage of seawater.
Answer:
It is a house of table salt (common salt).
Question 41.
What are the two types of water resources?
Answer:
1. Freshwater resources.
2. Saltwater (sea) resources.
Question 42.
What are the sources of freshwater?
Answer:
- Rainwater
- Surface water
- Groundwater
- Polar ice caps
- Ponds and Pools
Question 43.
What do you understand by aquatic habitat?
Answer:
Organisms which are found in water possess aquatic habitat.
Question 44.
How much percent of nitrogen is present in atmosphere?
Answer:
78%.
Question 45.
How is nitrogen used in living organisms.
Answer:
Nitrogen is a component of proteins, nucleic acid (DNA and RNA).
Question 46.
Name two plants which are capable of fixing atmospheric nitrogen.
Answer:
Green pea and other leguminous plants.
![]()
Question 47.
Write two uses of mirco-organisms.
Answer:
Micro-organisms act as biofertilizers. They also produce antibiotics.
Question 48.
Name any two natural cycles operating in nature.
Answer:
1. Carbon cycle
2. Nitrogen cycle
Question 49.
Name the gaseous components of biosphere.
Answer:
CO2, O2 and Nitrogen.
Question 50.
Name the source of energy for the process of photosynthesis.
Answer:
Solar energy.
Question 51.
Define biomass.
Answer:
The total weight of a living organism.
Question 52.
Name two nitrifying bacteria.
Answer:
Nitrosomonas and Nitrobacter.
Question 53.
Define pollution.
Answer:
Pollution is an undesirable change in physical, chemical or biological characteristics of air, water or land caused by pollutants.
![]()
Question 54.
What are pollutants?
Answer:
The substances causing pollution are termed pollutants.
Question 55.
What are the three major types of pollution?
Answer:
Water, air and soil pollution.
Question 56.
What is soil pollution?
Answer:
Soil pollution is removal of useful components from soil and addition of other substances which adversely affect the soil is termed soil pollution.
Question 57.
Which part of solar radiation is absorbed by ozone layer?
Answer:
UV rays.
Question 58.
Name the major surface water pollutant from farm run off and bathroom water.
Answer:
Phosphorus.
Question 59.
Give the source of pathogens in the water.
Answer:
Domestic sewage.
Question 60.
What is the source of aerosols?
Answer:
Aeroplanes.
Question 61.
Which term is used for pollutants that are degraded by natural means?
Answer:
Biodegradable.
![]()
Question 62.
How can SO2 pollution of air be checked?
Answer:
By using sulphur-free fuel in automobiles.
Question 63.
Mention the regions where rainfall is highest and lowest in India.
Answer:
- Minimum rainfall: Arid region having rainfall of 20 to 50 cm.
- Maximum rainfall: Wet region having rainfall of more than 200 cm.