Punjab State Board PSEB 11th Class Chemistry Important Questions Chapter 7 Equilibrium Important Questions and Answers.
PSEB 11th Class Chemistry Important Questions Chapter 7 Equilibrium
Very Short Answer Type Questions
Question 1.
A tank is full of water. Water is coming in as well as going out at same rate. What will happen to level of water in a tank? What is name given to such state?
Answer:
It will remain the same because rate of inflow is equal to rate of outflow. This state is called state of ‘equilibrium’.
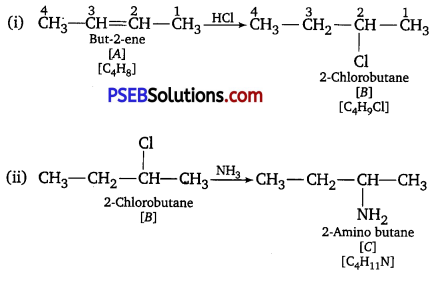
Question 2.
The ionization of hydrogen chloride in water is given t
HCl(aq) + H2O(l) ⇌ H3O++(aq) + Cl–(aq)
Label two conjugate acid-base pairs in this ionization.
Answer:
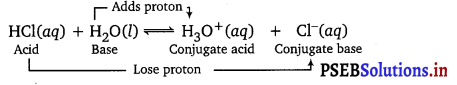
![]()
Question 3.
Why solution of sugar in water does not conduct electricity whereas that of common salt in water does?
Answer:
Common salt (NaCl) is an electrolyte which gives Na+ and Cl– ions in the aqueous solution. Hence, it conducts electricity. Sugar is sucrose (C12H22O11) which is a non-electrolyte and does not give ions in the solution. Hence, it does not conduct electricity.
Question 4.
Why is ammonia termed as a base though it does not contain OH– ions?
Answer:
Ammonia is termed as a base due to its tendency to donate electron pair. Therefore it is a Lewis base.
Question 5.
Kb for NH4O, H is 1.8 x 10-5 and for CH3NH2 is 44 x 10-4. Which of them is strongest base and why?
Answer:
CH3NH2 is strongest base because it has high value of base dissociation constant.
Question 6.
pKa value of acids A, B, C, D are 1.5, 3.5, 2.0 and 5.0. Which of them is strongest acid?
Answer:
Acid A with pKa = 1.5 is strongest acid, lower the value of pKa stronger will be the acid.
Question 7.
What will be the pH of 1M Na2SO4 solution?
Answer:
Na2SO4 is salt of strong acid and strong base, thus its aqueous solution will be neutral. Therefore, its pH will be 7.
Question 8.
Is it possible to get precipitate of Fe(OH)3 at pH = 2? Give reason.
Answer:
No, because Fe(OH)3 will dissolve in strongly acidic medium.
![]()
Question 9.
What happens to ionic product of water if some acid is added to it?
Answer:
Ionic product will remain unchanged.
Question 10.
How does common ion affect the solubility of electrolyte?
Answer:
Solubility of electrolyte decreases due to common ion effect.
Short Answer Type Questions
Question 1.
A certain buffer is made by mixing sodium form ate and formic acid in water. With the help of equations explain how this buffer neutralises addition of a small amount of an acid or a base?
Answer:
HCOONa → HCOO– + Na+
HCOOH ⇌ HCOO– + H+
HCOO– is common ion in the above acidic buffer. When small amount of H+ ions is added, these H+ ions combine with HCOO– which are in excess to form HCOOH back and [H+] remains practically same, so pH remains constant. When small amount of OH– ions are added, OH– ions will take up H+ and association of HCOOH will increase so as to maintain concentration of H+ ions. So, pH would not be affected.
Question 2.
How much volume of 0.1 M CH3COOH should he added to 50 ml of 0.2 M CH3COONa solution to prepare a buffer solution of pH 4.91. (pAa of AcH is 4.76).
According to Henderson’s equation
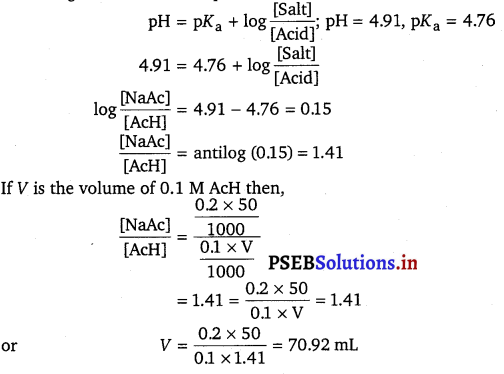
Required volume of 0.1 M acetic acid = 70.92 mL
Question 3.
Some processes are given below. What happens to the process if it is subjected to a change given in the brackets?
![]()
(ii) Dissolution of NaOH in water (Temperature is increased)
(iii) N2(g) + O2(g) ⇌ 2NO(g) -180.7 kJ (Pressure is increased and temperature is decreased.)
Answer:
(i) Equilibrium will shift in the forward direction, i.e., more ice will melt.
(ii) Solubility will decrease because it is an exothermic process.
(iii) Pressure has no effect. Decrease of temperature will shift the equilibrium in the backward direction.
![]()
Question 4.
50.0 g of CaCO3 are heated to 1073 K in a 5 L vessel. What percent of the CaCO3 would decompose at equilibrium? Kp for the reaction CaCO3(s) ⇌ CaO(s) + CO2(g) is 1.15 atm at 1073 K.
Answer:
The reaction is : CaCO3(s) ⇌ CaO(s) + CO2(g)
Kp = PCo2 = 1.15 atm, pV = nRT
\(\mathrm{n}_{\mathrm{CO}_{2}}=\frac{p_{\mathrm{CO}_{2}} \mathrm{~V}}{R T}=\frac{1.15 \times 5}{0.082 \times 1073}\) = 0.065 mol
1 mole of CO2 is obtained by decomposition of 1 mole CaCO3. Therefore, moles of CaCO3 decomposed is equal to the moles of CO2 = 0.065 mol.
Mole of CaCO3 initially present = \(\frac{50}{100}\) = 0.5 mol
[Molecular mass of CaCO3 = 100]
Per cent of CaCO3 decomposed = \(\frac{0.065}{0.5}\) x 100 = 13%
Question 5.
Arrange the following in increasing order of pH.
KNO3(aqr), CH3COONa(aq), NH4Cl(aq), C6H5COONH4(aq)
Answer:
(i) KNO3 is a salt of strong acid-strong base, hence its aqueous solution is neutral; pH = 7
(ii) CH3COONa is a salt of weak acid and strong base, hence, its aqueous solution is basic; pH < 7.
(iii) NH4Cl is a salt of strong acid and weak base, hence its aqueous solution is acidic; pH < 7.
(iv) C6H5COONH4 is a salt of weak acid, C6H5COOH and weak base, NH4OH. ButNH4OH is slightly stronger than C6H5COOH. Hence, pH is slightly greater than 7.
Therefore, increasing order of pH of the given salts is,
NH4Cl < KNO3 < C6H5COONH4 < CH3COONa
Long Answer Type Questions
Question 1.
Calculate the pH of a buffer which is 0.1 M in acetic acid and 0.15 M in sodium acetate. Given that the ionisation constants of acetic acid is 1.75 x 10-5. Also calculate the change in pH of the buffer if the following adds in 1 L of the buffer (i) 1 cc of 1 M NaOH. (ii) 1 cc of 1 M HC1. Assume that the charge in volume is negligible, (iii) What will be the buffer index of the above buffer?
Answer:
pH = pKa + log\(\frac{Salt}{Acid}\) = – log(1.75 x 10-5) + log
\(\frac{0.15}{0.10}\)
= (5 – 0.2430) + 0.1761 = 4.757 + 0.1761 = 4.933.
(i) 1 cc of 1M NaOH contains NaOH = 10-3 mol. This will convert 10-3 mol of acetic acid into the salt so that salt formed = 10-3 mol.
[Acid] = 0.10 – 0.001 = 0.099 M
[Salt] = 0.15 + 0.001 = 0.151 M
pH =. 4.757 + log \(\frac{0.151}{0.099}\)
= 4.757 + 0.183 = 4.940
∴ Increase in pH = 4.940 – 4.933 = 0.007 which is negligible.
(ii) 1 cc of 1 M HC1 contains HCl = 1CF3 mol. This will convert 10-3 mol CH3COONa into CH3COOH.
Now, [Acid] = 0.10 + 0.001 = 0.101 M
[Salt] = 0.15 – 0.001 = 0.149 M 0.149
∴ pH = 4.757 + log\(\frac{0.149}{0.101}\) = 4.757 + 0.169 = 4.925
∴ Decrease in pH = 4.933 = 0.007 which is again negligible.
(iii) Calculation of buffer index No. of moles of HC1 or NaOH added = 0.001 mol
Change in pH = 0.007
Hence, buffer index = \(\frac{\Delta n}{\Delta \mathrm{pH}}=\frac{0.001}{0.007}=\frac{1}{7}\)= 0.143
Question 2.
On the basis of Le-Chatelier’s principle, explain how temperature and pressure can be adjusted to increase the yield of ammonia in the following reaction?
N2(g) + 3H2(g) ⇌ 2NH3(g)
What will be the effect of addition of argon to the above reaction mixture at constant volume?
Answer:
N2(g) + 3H2(g) ⇌ 2NH3(g); ΔH = -92.38 kJ mol-1
![]()
It is an exothermic process. According to Le-Chatelier’s principle, low temperature is favourable for high yield of ammonia, but practically very low temperatures slow down the reaction. So, optimum temperature, 700 K is favourable in attainment of equilibrium.
Similarly, high pressure about 200 atm is favourable for high yield of ammonia. On increasing pressure, reaction goes in the forward direction because the number of moles decreases in the forward direction.
At constant volume, addition of argon does not affect the equilibrium because it does not change the partial pressures of the reactants or products involved in the reaction and the equilibrium remains undisturbed.
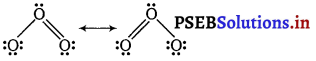
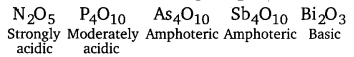
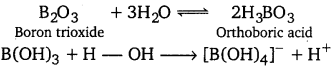
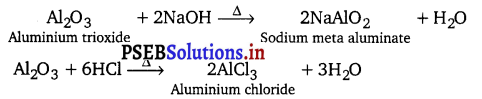
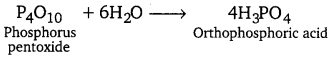
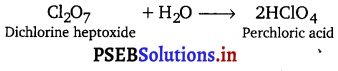
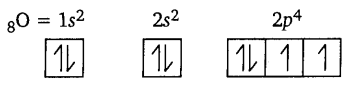
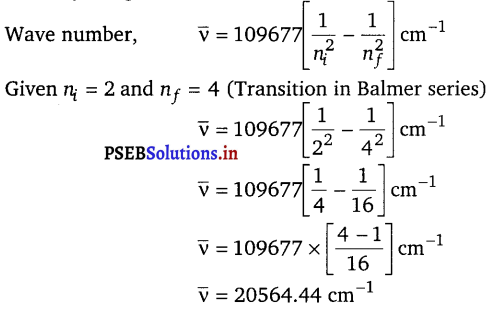
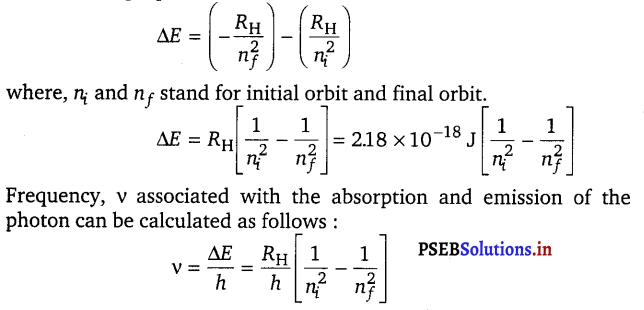
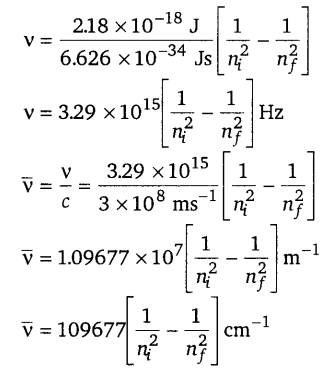
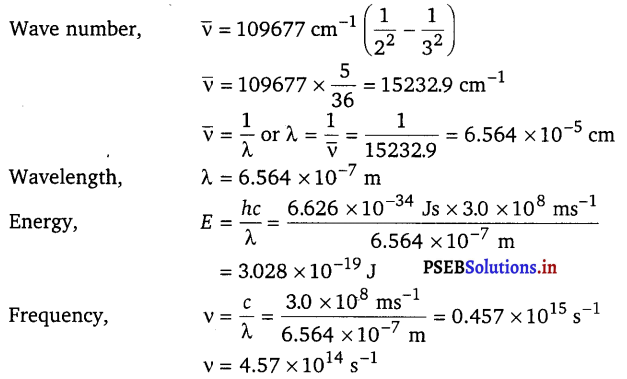
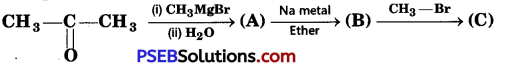
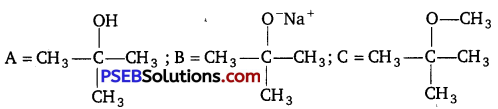
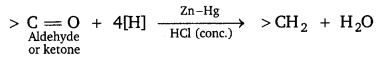

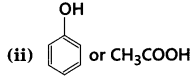

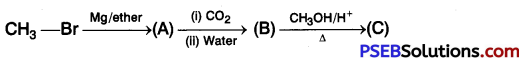
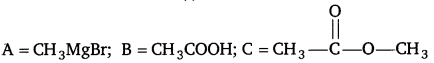
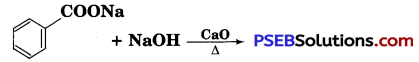
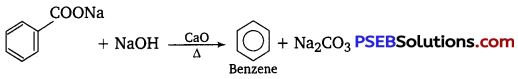
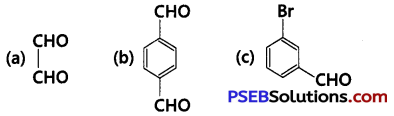
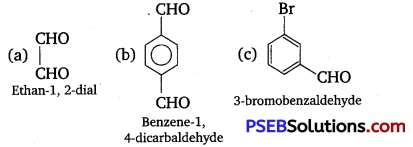
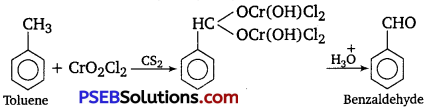
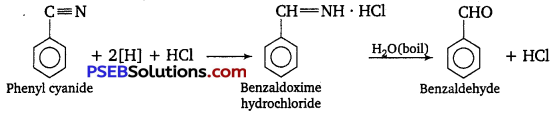
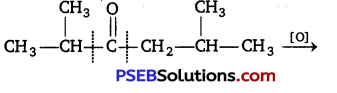
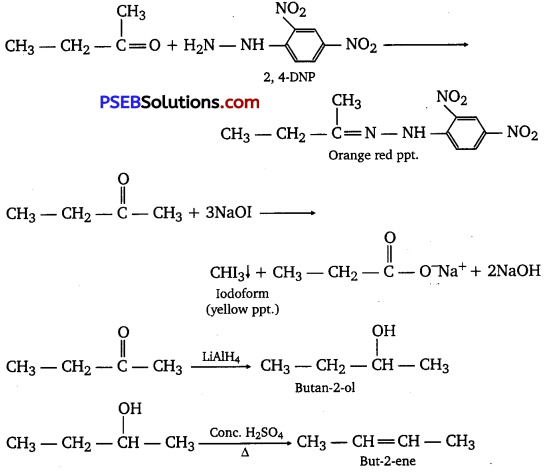
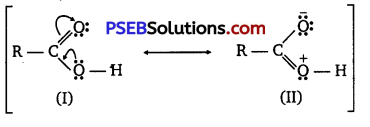
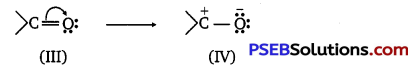
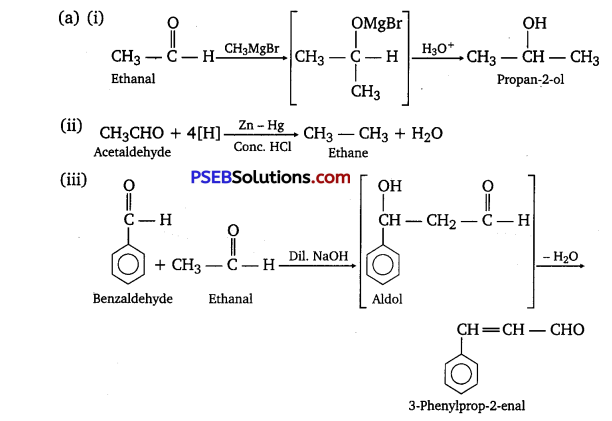
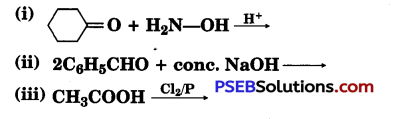
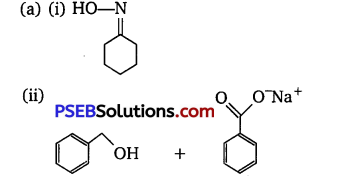
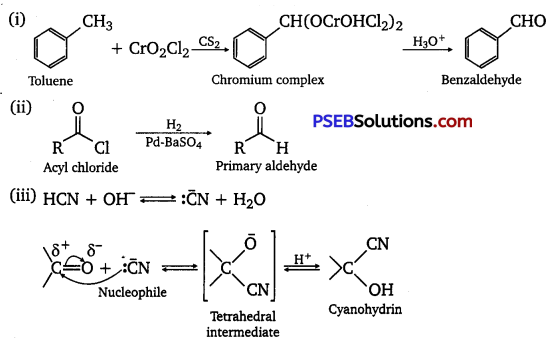

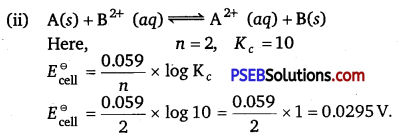
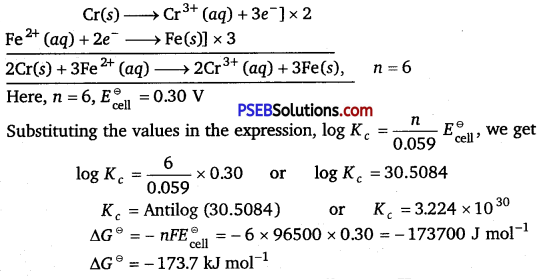

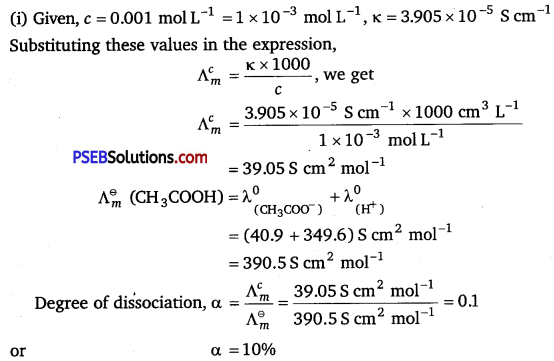
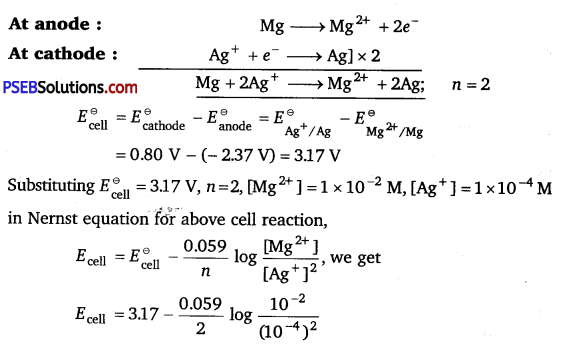
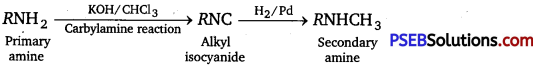
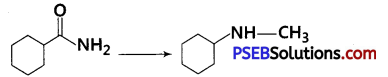
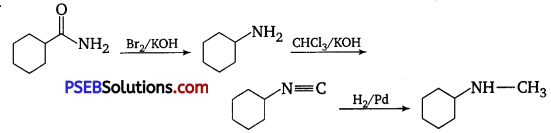
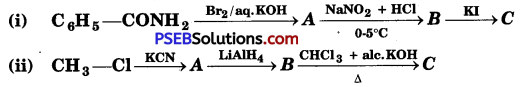
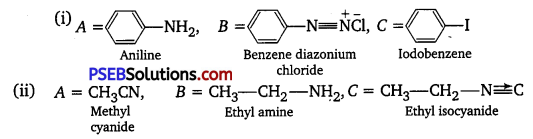
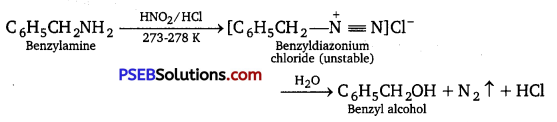
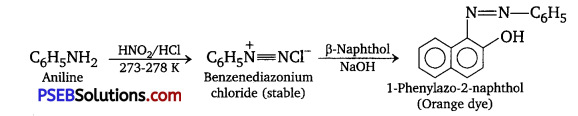
 [NCERT Exemplar]
[NCERT Exemplar]