Punjab State Board PSEB 12th Class Chemistry Important Questions Chapter 2 Solutions Important Questions and Answers.
PSEB 12th Class Chemistry Important Questions Chapter 2 Solutions
Very short answer type questions
Question 1.
Define mole fraction.
Answer:
Mole fraction of a component in a solution may be defined as the ratio of moles of that compdnent to the total number of moles of all the components present in the solution.
Question 2.
What is the similarity between Raoult’s law and Henry’s law?
Answer:
The similarity between Raoult’s law and Henry’s law is that both state that the partial vapour pressure of the volatile component or gas is directly proportional to its mole fraction in the solution.
Question 3.
Why is the vapour pressure of a solution of glucose in water lower than that of water? (NCERT Exemplar)
Answer:
This is due to decrease in the escaping tendency of the water molecules from the surface of solution as some of the surface area is occupied by non-volatile solute, glucose particles.
![]()
Question 4.
What type of deviation is shown by a mixture of ethanol and acetone? What type of azeotrope is formed by mixing ethanol and acetone?
Answer:
Positive deviation.
Minimum boiling azeotropes.
Question 5.
State how does osmotic pressure vary with temperature.
Answer:
Osmotic pressure increases with increase in temperature.
Question 6.
What are isotonic solutions?
Answer:
The solutions of the same osmotic pressure at a given temperature are called isotonic solutions.
Question 7.
Define van’t Hoff factor.
Answer:
van’t Hoff factor may be defined as the ratio of normal molecular mass to observed molecular mass or the ratio of observed colligative property to normal colligative property.
Question 8.
Why are aquatic species more comfortable in cold water in comparison to warm water? (NCERT Exemplar)
Answer:
Solubility of oxygen in water increases with decrease in temperature. Presence of more oxygen at lower temperature makes the aquatic species more comfortable in cold water.
![]()
Question 9.
What is semipermeable membrane? (NCERT Exemplar)
Answer:
Continuous sheets or films (natural or synthetic) which contain a network of submicroscopic holes or pores through which small solvent molecules (water etc.) can pass, but solute molecules of bigger size cannot pass are called semipermeable membrane.
Question 10.
Give an example of a material used for making semipermeable membrane for carrying out reverse osmosis. (NCERT Exemplar)
Answer:
Cellulose acetate.
Short answer type questions
Question 1.
State Henry’s law. Write its one application. What is the effect of temperature on solubility of gases in liquid?
Answer:
It states that the partial pressure of a gas in vapour phase (p) is proportional to the mole fraction of the gas (χ) in the solution.
p ∝ χ or p = KHχ where KH is the
Henry’s constant.
Application of Henry’s law:
To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure.
Effect of temperature on solubility:
As dissolution is an exothermic process, therefore, according to Le Chatelier’s principle solubility should decrease with rise in temperature.
Question 2.
Why does a solution containing non-volatile solute have higher boiling point than the pure solvent? Why is elevation of boiling point a colligative property?
Answer:
The addition of a non-volatile solute to a volatile solvent lowers its vapour pressure. In order to boil the solution, i.e., to make its vapour pressure equal to atmospheric pressure, the solution has to be heated at a higher temperature. In other words, the boiling point of solution becomes higher than solvent.
As elevation in boiling point depends on the number of moles of solute particles and independent of their nature, therefore, it is a colligative property.
![]()
Question 3.
Will the elevation in boiling point be same if 0.1 mol of sodium chloride or 0.1 mol of sugar is dissolved in 1L of water? Explain.
Answer:
No, the elevation in boiling point is not the same. NaCl, being an electrolyte, dissociates almost completely to give Na+ and Cl– ions whereas glucose, being non-electrolyte does not dissociate. Hence, the number of particles in 0.1 M NaCl solution is nearly double than 0.1 M glucose solution. Elevation in boiling point being a colligative property, is therefore, nearly twice for 0.1 M NaCl solution than for 0.1 M glucose solution.
Question 4.
Explain the solubility rule ‘like dissolves like’ in terms of intermolecular forces that exist in solutions. (NCERT Exemplar)
Answer:
If the intermolecular interactions are similar in both constituents, i.e., solute and solvent then solute dissolves in the solvent. e.g., polar solutes dissolve in polar solvents and non-polar solutes in non-polar solvents. Thus, the statement ‘like dissolved like’ proves to be true.
Question 5.
Concentration terms such as mass percentage, ppm, mole fraction and molality are independent of temperature. However, molarity is a function of temperature. Explain.
(NCERT Exemplar)
Answer:
Molarity is defined as the number of moles of solute dissolved per litre of a solution. Since, volume depends on temperature and changes with change in temperature, the molarity will also change with change in temperature. On the other hand, mass does not change with change in temperature, so other concentration terms given in the question also do not do so. According to the definition of all these terms, mass of solvent used for making the solution is related to the mass of solute.
![]()
Question 6.
Which of the following solutions has higher freezing point?
0.05 M Al2(SO4)3, 0.1 M K3 [Fe(CN)6] Justify.
Answer:
0.05 M Al2(SO4)3 has higher freezing point.
Justification:
For 0.05 MAl2(SO4) 3,i = 5
Number of particles = i × concentration
= 5 × 0.05
= 0.25 moles of ions
For 0.1MK3 [Fe(CN)6], i = 4
Number of particles = i × concentration
= 4 × 0.1
= 0.4 moles of ions
We know that, ΔTf. Number of particles
Hence, 0.05 M Al2(SO4)3 has higher freezing point because it has lower number of particles than 0.1 M K3 [Fe (CN)6].
Question 7.
The freezing point of benzene decreases by 2.12 K when 2.5 g of benzoic acid (C6H5COOH) is dissolved in 25 g of benzene. If benzoic acid forms a dimer in benzene, calculate the van’t Hoff factor and the percentage association of benzoic acid. (Kf for benzene = 5.12 K kg mol-1)
Answer:
Given, ΔTf = 2.12 K, Kf = 5.12 K kg mol-1
We know that, ΔTf = i Kf m
2.12 = \(\frac{i \times 5.12 \times 2.5 \times 1000}{122 \times 25}\)
or i = 0.505
For association,
i = 1 – \(\frac{\alpha}{2}\)
0.505 = 1 – \(\frac{\alpha}{2}\)
or α =0.99
Hence, percentage association of benzoic acid is 99%.
Long answer type questions
Question 1.
(a) What type of deviation is shown by a mixture of ethanol and acetone? Give reason.
(b) A solution of glucose (molar mass = 180 g mol-1) in water is labelled as 10% (by mass). What would be the molality and molarity of the solution?
(Density of solution = 1.2 g mL-1)
Answer
(a) A mixture of ethanol and acetone shows positive deviation from Raoult’s law.
In pure ethanol hydrogen bond exist between the molecules. On adding acetone to ethanol, acetone molecules get in between the molecules of . ethanol thus breaking some of the hydrogen bonds and weakening molecular interactions considerably. Weakening of molecular interactions leads to increase in vapour pressure resulting in positive deviation from Raoult’s law.
(b) Let the mass of solution = 100 g
∴ Mass of glucose = 10 g
Number of moles of glucose \(=\frac{\text { Mass of glucose }}{\text { Molar mass }}\)
= \(\frac{10 \mathrm{~g}}{180 \mathrm{~g} \mathrm{~mol}^{-1}}\) = 0.056 mol
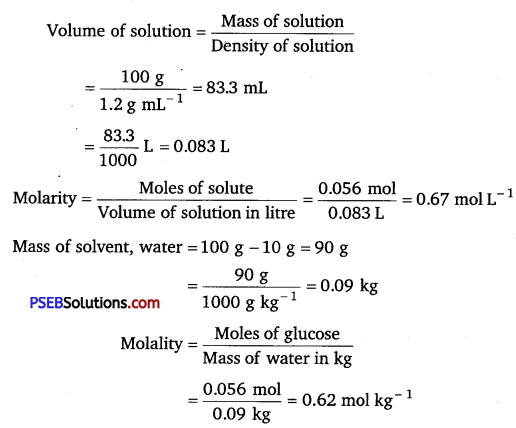
![]()
Question 2.
Discuss biological arid industrial importance of osmosis.
(NCERT Exemplar)
Answer:
The process of osmosis is of great biological and industrial importance as is evident from the following examples:
- Movement of water from soil into plant roots and subsequently into upper portion of the plant occurs partly due to osmosis.
- Preservation of meat against bacterial action by adding salt.
- Preservation of fruits against bacterial action by adding sugar.
Bacterium in canned fruit loses water through the process of osmosis, shrivels and dies. - Reverse osmosis is used for desalination of water.