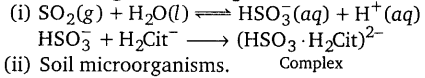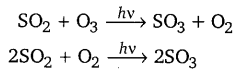Punjab State Board PSEB 11th Class Environmental Education Important Questions Chapter 3 ਮਨੁੱਖੀ ਗਤੀਵਿਧੀਆਂ ਦਾ ਵਾਤਾਵਰਣ ਉੱਪਰ ਪ੍ਰਭਾਵ Important Questions and Answers.
PSEB 11th Class Environmental Education Important Questions Chapter 3 ਮਨੁੱਖੀ ਗਤੀਵਿਧੀਆਂ ਦਾ ਵਾਤਾਵਰਣ ਉੱਪਰ ਪ੍ਰਭਾਵ
(ਉ) ਬਹੁਤ ਛੋਟੇ ਉੱਤਰਾਂ ਵਾਲੇ ਪ੍ਰਸ਼ਨ
ਪ੍ਰਸ਼ਨ 1.
ਅਮਲੀ ਵਰਖਾ/ਤੇਜ਼ਾਬੀ ਵਰਖਾ (Acid Rain) ਦੇ ਕਾਰਨ ਕੀ ਹੈ ?
ਉੱਤਰ-
ਵਾਹਨਾਂ ਅਤੇ ਕਾਰਖਾਨਿਆਂ ਵਿਚੋਂ ਨਿਕਲਣ ਵਾਲੇ ਧੂੰਏਂ ਵਿਚ ਸਲਫਰ ਡਾਈ ਆਕਸਾਈਡ ਨਾਂ ਦੀ ਗੈਸ ਹੁੰਦੀ ਹੈ ਜਿਹੜਾ ਕਿ ਵਾਯੂਮੰਡਲ ਵਿਚ ਸ਼ਾਮਿਲ ਪਾਣੀ ਦੇ ਵਾਸ਼ਪਾਂ ਨਾਲ ਮਿਲ ਕੇ ਸਲਫਿਊਰਿਕ ਅਮਲ ਦਾ ਨਿਰਮਾਣ ਕਰਦੀ ਹੈ । ਇਸ ਦੇ ਕਾਰਨ ਹੀ ਅਮਲੀ/ ਤੇਜ਼ਾਬੀ ਵਰਖਾ ਹੁੰਦੀ ਹੈ ।
ਪ੍ਰਸ਼ਨ 2.
ਜੰਗਲਾਂ ਦੇ ਖ਼ਾਤਮੇ ਦਾ ਮੁੱਖ ਕਾਰਨ ਕੀ ਹੈ ?
ਉੱਤਰ-
ਉਦਯੋਗੀਕਰਨ, ਸ਼ਹਿਰੀਕਰਨ ਅਤੇ ਜੰਗਲਾਂ ਨੂੰ ਕੱਟਣਾ ਆਦਿ ਜੰਗਲਾਂ ਦੇ ਖਾਤਮੇ ਦੇ ਮੁੱਖ ਕਾਰਨ ਹਨ ।
ਪ੍ਰਸ਼ਨ 3.
ਉਦਯੋਗ ਦੇ ਲਈ ਖੋਜ ਕੀਤੀਆਂ ਗਈਆਂ ਨਵੀਆਂ ਅਤੇ ਕੁਸ਼ਲ ਵਿਧੀਆਂ ਦੇ ਨਾਮ ਦੱਸੋ ।
ਉੱਤਰ-
ਨੈਨੋ-ਉਦਯੋਗਿਕੀਕਰਨ, ਜੈਵ-ਪ੍ਰੋਉਦਯੋਗਿਕੀਕਰਨ ਅਤੇ ਜੈਵ-ਸੂਚਨਾ ਤਕਨਾਲੋਜੀ ।
ਪ੍ਰਸ਼ਨ 4.
ਨਾਗਰਿਕਤਾ ਸੰਬੰਧੀ ਸਹੂਲਤਾਂ/ਵਿਕ ਐਮਿਨਿਟੀ (Civic Amenities) ਦਾ ਕੀ ਅਰਥ ਹੈ ?
ਉੱਤਰ-
ਨਾਗਰਿਕਤਾ ਸੰਬੰਧੀ ਸਹੂਲਤਾਂ/ਸਿਵਿਕ ਐਮਿਨਿਟੀ ਤੋਂ ਅਰਥ ਨਗਰਾਂ ਦੀਆਂ ਸੁਵਿਧਾਵਾਂ ਤੋਂ ਹੈ ਜਿਹੜੀਆਂ ਨਗਰ ਪ੍ਰਸ਼ਾਸਨ ਦੁਆਰਾ ਸ਼ਹਿਰਾਂ ਅਤੇ ਕਸਬਿਆਂ ਵਿਚ ਰਹਿਣ ਵਾਲੇ ਲੋਕਾਂ ਦਾ ਜੀਵਨ ਅਰਾਮਦਾਇਕ ਬਣਾਉਣ ਦੇ ਲਈ ਪ੍ਰਦਾਨ ਕੀਤੀਆਂ ਜਾਂਦੀਆਂ ਹਨ ।
![]()
ਪ੍ਰਸ਼ਨ 5.
ਨਗਰ ਪ੍ਰਸ਼ਾਸਨ ਦੁਆਰਾ ਨਾਗਰਿਕਾਂ ਨੂੰ ਕਿਹੜੀਆਂ-ਕਿਹੜੀਆਂ ਸੁਵਿਧਾਵਾਂ ਦਿੱਤੀਆਂ ਜਾਂਦੀਆਂ ਹਨ ?
ਉੱਤਰ-
ਪੀਣ ਲਈ ਸਾਫ਼ ਪਾਣੀ, ਬਿਜਲੀ ਦਾ ਸਹੀ ਪ੍ਰਬੰਧ, ਡਾਕਟਰੀ ਸਹੂਲਤਾਂ ਆਦਿ ।
ਪ੍ਰਸ਼ਨ 6.
ਸ਼ਹਿਰੀਕਰਨ ਨੂੰ ਉਤਸ਼ਾਹ ਦੇਣ ਵਾਲੇ ਕਾਰਕ ਕਿਹੜੇ ਹਨ ?
ਉੱਤਰ-
ਆਬਾਦੀ ਵਿਚ ਵਾਧਾ, ਉਦਯੋਗੀਕਰਨ ਅਤੇ ਹੋਰ ਵਿਕਾਸਸ਼ੀਲ ਗਤੀਵਿਧੀਆਂ ।
ਪ੍ਰਸ਼ਨ 7.
ਚਲਦੀ-ਫਿਰਦੀ ਵਸੋਂ ਵਿਚ ਕਿਹੜੇ ਲੋਕ ਸ਼ਾਮਿਲ ਹੁੰਦੇ ਹਨ ?
ਉੱਤਰ-
ਚਲਦੀ-ਫਿਰਦੀ ਵਸੋਂ ਵਿਚ ਉਹ ਲੋਕ ਸ਼ਾਮਿਲ ਹੁੰਦੇ ਹਨ ਜਿਹੜੇ ਹਰ ਰੋਜ਼ ਆਪਣੀ ਰੋਜ਼ੀ-ਰੋਟੀ ਕਮਾਉਣ ਲਈ ਇਕ ਸਥਾਨ ਤੋਂ ਦੂਜੇ ਸਥਾਨ ‘ਤੇ ਜਾਂਦੇ ਹਨ ।
ਪ੍ਰਸ਼ਨ 8.
ਸ਼ਹਿਰੀਕਰਨ ਦਾ ਵਾਤਾਵਰਣ ਉੱਪਰ ਕੀ ਪ੍ਰਭਾਵ ਪੈਂਦਾ ਹੈ ?
ਉੱਤਰ-
ਸ਼ਹਿਰੀਕਰਨ ਵਾਤਾਵਰਣ ਦੀਆਂ ਭੌਤਿਕ, ਰਸਾਇਣਿਕ ਅਤੇ ਜੈਵਿਕ ਵਿਸ਼ੇਸ਼ਤਾਵਾਂ ਨੂੰ ਨੁਕਸਾਨ ਪਹੁੰਚਾਉਂਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 9.
ਰਸਾਇਣਿਕ ਖਾਦਾਂ ਦਾ ਜ਼ਿਆਦਾ ਪ੍ਰਯੋਗ ਕਰਨ ਨਾਲ ਭੂਮੀ ਨਾਲ ਸੰਬੰਧਿਤ ਕਿਹੜੀਆਂ ਸਮੱਸਿਆਵਾਂ ਪੈਦਾ ਹੁੰਦੀਆਂ ਹਨ ?
ਉੱਤਰ-
ਭੂਮੀ ਦੀ ਉਪਜਾਊ ਸ਼ਕਤੀ ਵਿਚ ਕਮੀ, ਰੇਗਿਸਤਾਨੀਕਰਨ ਅਤੇ ਭੋਂ-ਖੋਰਨ ਆਦਿ ।
ਪ੍ਰਸ਼ਨ 10.
ਕੁੱਝ ਸ਼ਹਿਰੀ ਸਮੱਸਿਆਵਾਂ ਦੇ ਨਾਂ ਲਿਖੋ ।
ਉੱਤਰ-
ਪ੍ਰਦੂਸ਼ਣ, ਗੰਦੀਆਂ ਬਸਤੀਆਂ ਦਾ ਵਿਕਾਸ, ਪਾਣੀ ਅਤੇ ਬਿਜਲੀ ਦੀਆਂ ਸਮੱਸਿਆਵਾਂ ਆਦਿ ।
ਪ੍ਰਸ਼ਨ 11.
ਪੇਂਡੂ ਖੇਤਰਾਂ ਨਾਲ ਸੰਬੰਧਿਤ ਸਮੱਸਿਆਵਾਂ ਦੇ ਨਾਂ ਲਿਖੋ ।
ਉੱਤਰ-
ਸਿੱਖਿਆ ਅਤੇ ਡਾਕਟਰੀ ਸਹੂਲਤਾਂ ਦੀ ਕਮੀ, ਸਫ਼ਾਈ ਅਤੇ ਜਲ-ਨਿਕਾਸ ਪ੍ਰਣਾਲੀ ਦੀ ਕਮੀ |
![]()
ਪ੍ਰਸ਼ਨ 12.
ਕੁਦਰਤੀ ਸੰਪੱਤੀ ਦੇ ਵਿਘਟਨ ਦੇ ਮੁੱਖ ਕਾਰਨ ਕੀ ਹਨ ?
ਉੱਤਰ-
ਆਬਾਦੀ ਵਿਚ ਵਾਧਾ, ਸਾਧਨਾਂ ਦਾ ਸਹੀ ਢੰਗ ਨਾਲ ਉਪਯੋਗ ਨਾ ਕਰਨਾ ਅਤੇ ਉਦਯੋਗਿਕ ਵਿਕਾਸ ਆਦਿ ।
ਪ੍ਰਸ਼ਨ 13.
ਪਾਣੀ ਪ੍ਰਦੂਸ਼ਣ ਦੇ ਕੀ ਕਾਰਨ ਹਨ ?
ਉੱਤਰ-
ਪਾਣੀ ਦਾ ਪ੍ਰਦੂਸ਼ਣ ਖੇਤੀ ਦੇ ਕੰਮਾਂ, ਉਦਯੋਗਾਂ ਅਤੇ ਘਰੇਲੂ ਵਿਅਰਥ ਪਦਾਰਥਾਂ ਦੁਆਰਾ ਹੁੰਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 14.
ਸੰਸਾਰ ਵਿਚ ਸਾਰਿਆਂ ਤੋਂ ਜ਼ਿਆਦਾ ਅਤੇ ਸਭ ਤੋਂ ਘੱਟ ਸ਼ਹਿਰੀਕਰਨ ਵਾਲੇ ਦੇਸ਼ਾਂ ਦੇ ਨਾਂ ਲਿਖੋ ।
ਉੱਤਰ-
ਸਭ ਤੋਂ ਜ਼ਿਆਦਾ ਸ਼ਹਿਰੀਕਰਨ ਵਾਲਾ ਦੇਸ਼ ਇਜ਼ਰਾਈਲ (917) ਅਤੇ ਸਭ ਤੋਂ ਘੱਟ ਸ਼ਹਿਰੀਕਰਨ ਵਾਲਾ ਦੇਸ਼ ਇਥੋਪੀਆ (13%) ਹੈ ।
ਪ੍ਰਸ਼ਨ 15.
ਪਾਣੀ ਦਬਾਉ ਕਿਸ ਨੂੰ ਕਹਿੰਦੇ ਹਨ ?
ਉੱਤਰ-
ਪਾਣੀ ਦਬਾਉ ਉਸ ਅਵਸਥਾ ਨੂੰ ਕਹਿੰਦੇ ਹਨ ਜਦੋਂ ਹਰ ਸਾਲ ਮਨੁੱਖ ਦੇ ਲਈ ਸਾਫ਼ ਪਾਣੀ ਦੀ ਉਪਲੱਬਧ ਮਾਤਰਾ 1700 ਕਿਊਬਿਕ ਮੀਟਰ ਤੋਂ ਘੱਟ ਹੋ ਜਾਵੇ ।
ਪ੍ਰਸ਼ਨ 16.
ਆਵਾਜਾਈ ਸਾਧਨਾਂ ਦੇ ਵਧਣ ਨਾਲ ਹੋਣ ਵਾਲੇ ਮਾੜੇ ਪ੍ਰਭਾਵ ਕਿਹੜੇ ਹਨ ?
ਉੱਤਰ-
ਪੈਟਰੋਲੀਅਮ ਪਦਾਰਥਾਂ ਦੀ ਜ਼ਿਆਦਾ ਖ਼ਪਤ, ਵਾਯੂ ਪ੍ਰਦੂਸ਼ਣ, ਆਵਾਜਾਈ ਸੰਬੰਧੀ ਰੁਕਾਵਟਾਂ ਆਦਿ ।
ਪ੍ਰਸ਼ਨ 17.
ਗੰਦੀਆਂ ਬਸਤੀਆਂ (Slums) ਦੇ ਵਿਕਾਸ ਹੋਣ ਦੇ ਮੁੱਖ ਕਾਰਨ ਕਿਹੜੇ ਹਨ ?
ਉੱਤਰ-
ਰਹਿਣ ਲਈ ਥਾਂ ਦੀ ਕਮੀ ਅਤੇ ਗਰੀਬੀ ।
ਪ੍ਰਸ਼ਨ 18.
ਸੰਸਾਧਨ ਉਪਭੋਗ ਦਰ (Resource Consumption Rate) ਕਿਸ ਨੂੰ ਕਹਿੰਦੇ ਹਨ ?
ਉੱਤਰ-
ਕਿਸੇ ਖਾਸ ਖੇਤਰ ਵਿਚ ਪ੍ਰਾਕਿਰਤਕ ਸੰਸਾਧਨਾਂ ਦੀ ਉਪਭੋਗਤਾ ਦੀ ਦਰ ਨੂੰ ਸੰਸਾਧਨ ਉਪਭੋਗਤਾ ਦਰ ਕਹਿੰਦੇ ਹਨ ।
ਪ੍ਰਸ਼ਨ 19.
ਠੋਸ ਕੂੜਾ-ਕਰਕਟ ਦੀਆਂ ਉਦਾਹਰਨਾਂ ਦਿਉ ।
ਉੱਤਰ-
ਮਿਊਂਸੀਪਲ ਕੂੜਾ-ਕਰਕਟ, ਘਰੇਲੂ ਕੂੜਾ-ਕਰਕਟ, ਉਦਯੋਗਿਕ ਕੂੜਾ-ਕਰਕਟ ਆਦਿ ।
ਪ੍ਰਸ਼ਨ 20.
ਰਾਸ਼ਟਰੀ ਵਣ ਨੀਤੀ (National Forest Policy) ਅਨੁਸਾਰ ਦੇਸ਼ ਦੇ ਕਿੰਨੇ ਹਿੱਸੇ ਉੱਪਰ ਵਣ ਹੋਣੇ ਚਾਹੀਦੇ ਹਨ ?
ਉੱਤਰ-
ਦੇਸ਼ ਦੀ ਕੁੱਲ ਭੂਮੀ ਦੇ 1/3 ਭਾਗ ਤੇ ਵਣ ਹੋਣੇ ਚਾਹੀਦੇ ਹਨ ।
ਪ੍ਰਸ਼ਨ 21.
ਗ੍ਰਾਮੀਣ ਖੇਤਰ ਦੀਆਂ ਸਮੱਸਿਆਵਾਂ ਕੀ ਹਨ ?
ਉੱਤਰ-
ਗਾਮੀਣ ਖੇਤਰ ਦੀਆਂ ਸਮੱਸਿਆਵਾਂ ਹਨ –
- ਵਿੱਦਿਆ ਦੀ ਘਾਟ,
- ਪੀਣ ਵਾਲੇ ਸ਼ੁੱਧ ਪਾਣੀ ਦੀ ਕਮੀ,
- ਪ੍ਰਦੂਸ਼ਣ,
- ਡਾਕਟਰੀ ਸਹੂਲਤਾਂ ਦੀ ਘਾਟ,
- ਫੋਕਟ ਪਦਾਰਥਾਂ ਦੇ ਨਿਪਟਾਰੇ ਦੀਆਂ ਸੁਵਿਧਾਵਾਂ ਦੀ ਘਾਟ ਆਦਿ ।
![]()
(ਅ) ਛੋਟੇ ਉੱਤਰਾਂ ਵਾਲੇ ਪ੍ਰਸ਼ਨ (Type I)
ਪ੍ਰਸ਼ਨ 1.
ਜੰਗਲਾਂ ਦੇ ਵਿਨਾਸ਼ ਦੇ ਪ੍ਰਭਾਵ ਦੱਸੋ ।
ਉੱਤਰ-
ਜੰਗਲਾਂ ਦੇ ਵਿਨਾਸ਼ ਦੇ ਪ੍ਰਭਾਵ ਹੇਠ ਲਿਖੇ ਅਨੁਸਾਰ ਹਨ –
- ਜੰਗਲੀ ਜੀਵਾਂ ਦੇ ਨਿਵਾਸ ਸਥਾਨਾਂ ਦਾ ਨਾਸ਼
- ਭੂ-ਖੋਰਨ
- ਤਾਪਮਾਨ ਵਿਚ ਵਾਧਾ ।
- ਜਲਵਾਯੂ ਵਿਚ ਪਰਿਵਰਤਨ
- ਹੜ੍ਹਾਂ ਦਾ ਆਉਣਾ।
ਪ੍ਰਸ਼ਨ 2.
ਪਾਣੀ ਦੇ ਜ਼ਿਆਦਾ ਉਪਯੋਗ ਹੋਣ ਦੇ ਕਾਰਨ ਦੱਸੋ ।
ਉੱਤਰ-
ਪਾਣੀ ਦੇ ਜ਼ਿਆਦਾ ਉਪਯੋਗ ਹੋਣ ਦੇ ਕਾਰਨ ਹੇਠ ਲਿਖੇ ਅਨੁਸਾਰ ਹਨ –
- ਵਸੋਂ ਵਿਸਫੋਟ
- ਉਦਯੋਗਿਕ ਗਤੀਵਿਧੀਆਂ
- ਸਿੰਜਾਈ ਦੇ ਲਈ ।
- ਘਰੇਲੂ ਵਰਤੋਂ ਲਈ।
ਪ੍ਰਸ਼ਨ 3.
ਜੰਗਲਾਂ ਦੇ ਜ਼ਿਆਦਾ ਕੱਟਣ ਦੇ ਮਾੜੇ ਪ੍ਰਭਾਵ ਦੱਸੋ ।
ਉੱਤਰ-
- ਵਣ ਵਿਨਾਸ਼
- ਭੋਂ-ਖੋਰ
- ਪਾਣੀ ਪ੍ਰਦੂਸ਼ਣ
- ਹਵਾ ਪ੍ਰਦੂਸ਼ਣ
- ਜੰਗਲੀ ਜੀਵਾਂ ਦੇ ਨਿਵਾਸ ਸਥਾਨਾਂ ਦਾ ਨਾਸ਼
- ਜਲ ਚੱਕਰ ਵਿਚ ਗੜਬੜ ਆਦਿ |
ਪ੍ਰਸ਼ਨ 4.
ਭੂਮੀਗਤ ਜਾਂ ਜ਼ਮੀਨ ਹੇਠਲਾ ਪਾਣੀ (Underground Water) ਕਿਵੇਂ ਦੂਸ਼ਿਤ ਹੁੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਖੇਤੀ-ਬਾੜੀ ਕਰਕੇ, ਘਰੇਲੂ ਰਹਿੰਦ-ਖੂੰਹਦ ਪਦਾਰਥ, ਕੂੜਾ-ਕਰਕਟ, ਉਦਯੋਗਾਂ ਦੇ ਵਿਕਾਸ, ਮਨੁੱਖੀ ਕਾਰ-ਵਿਹਾਰ, ਲਾਪਰਵਾਹੀ ਕੁਦਰਤੀ ਜਾਂ ਮਨੁੱਖੀ), ਕੁਦਰਤੀ ਅਤੇ ਗ਼ੈਰਕੁਦਰਤੀ ਰਸਾਇਣਿਕ ਕਿਰਿਆਵਾਂ ਆਦਿ ਪੱਧਰੀ ਪਾਣੀ ਨੂੰ ਦੂਸ਼ਿਤ ਕਰ ਰਹੇ ਹਨ । ਇਨ੍ਹਾਂ ਤੋਂ ਇਲਾਵਾ ਭੂਮੀ ਦੀ ਉਪਜਾਊ ਸ਼ਕਤੀ ਦੀ ਭਰਾਈ ਦੇ ਲਈ ਵਰਤੋਂ ਵਿਚ ਲਿਆਂਦੀਆਂ ਗਈਆਂ ਰਸਾਇਣਿਕ ਖਾਦਾਂ ਵੀ ਭੁਮੀ ਹੇਠਲੇ ਪਾਣੀ ਨੂੰ ਪ੍ਰਦੂਸ਼ਿਤ ਕਰਦੀਆਂ ਹਨ ।
ਪ੍ਰਸ਼ਨ 5.
ਖੇਤੀ-ਬਾੜੀ ਦੇ ਕੰਮਾਂ ਵਿਚ ਮਨੁੱਖੀ ਲਾਪਰਵਾਹੀ ਨਾਲ ਵਾਤਾਵਰਣ ‘ਤੇ ਕੀ ਅਸਰ ਹੁੰਦਾ ਹੈ ?
ਉੱਤਰ-
ਮਨੁੱਖ ਦੀਆਂ ਅਨਿਆਂ ਸੰਗਤ ਗਤੀਵਿਧੀਆਂ ਅਤੇ ਲਾਪਰਵਾਹੀਆਂ ਨਾਲ ਵਾਤਾਵਰਣ ‘ਤੇ ਮਾੜਾ ਅਸਰ ਪੈਂਦਾ ਹੈ । ਖੇਤੀ-ਬਾੜੀ ਦੇ ਕੰਮਾਂ ਵਿਚ ਇਹ ਲਾਪਰਵਾਹੀਆਂ ਵਧੇਰੇ ਹੁੰਦੀਆਂ ਹਨ, ਜਿਵੇਂ-ਖਾਦਾਂ ਦਾ ਜ਼ਿਆਦਾ ਮਾਤਰਾ ਵਿਚ ਉਪਯੋਗ, ਕੀਟਨਾਸ਼ਕਾਂ ਦੀ ਬੇਲੋੜੀ ਵਰਤੋਂ, ਖੇਤੀ-ਬਾੜੀ ਰਹਿੰਦ-ਖੂੰਹਦ ਦੀ ਸੰਭਾਲ ਨਾ ਕਰਨਾ ਆਦਿ । ਇਨ੍ਹਾਂ ਨਾਲ ਵਾਤਾਵਰਣ ਦੇ ਸਾਰੇ ਅੰਸ਼ਾਂ ਦਾ ਸੰਤੁਲਨ ਵਿਗੜ ਜਾਂਦਾ ਹੈ ਜਿਸ ਨਾਲ ਭੂਮੀ ਵਿਚ ਲੂਣਾਂ ਦਾ ਵਧਣਾ,ਮਰੁਸਥਲੀਕਰਨ, ਭੋਂ-ਖੁਰਨਾ ਅਤੇ ਪੈਦਾਵਾਰ ਦਾ ਘਟਣਾ ਮੁੱਖ ਹਨ ।
ਪ੍ਰਸ਼ਨ 6.
ਏਅਰ-ਕੰਡੀਸ਼ਨਰ ਦੀ ਵਰਤੋਂ ਦੇ ਕੀ ਨੁਕਸਾਨ ਹਨ ?
ਉੱਤਰ-
ਏਅਰ-ਕੰਡੀਸ਼ਨਰ ਦੀ ਵਰਤੋਂ ਗਰਮੀਆਂ ਦੇ ਮੌਸਮ ਵਿਚ ਬਿਜਲੀ ਦੀ ਘਾਟ ਪੈਦਾ ਕਰਦੀ ਹੈ । ਇਸ ਨਾਲ ਬਿਜਲੀ ਦੇ ਕੱਟ ਲੱਗਦੇ ਹਨ । ਕੰਮ-ਕਾਜ ਠੱਪ ਹੋ ਜਾਣ ਤੋਂ ਰੋਕਣ ਲਈ ਜਰਨੇਟਰ ਦੀ ਵਰਤੋਂ ਕੀਤੀ ਜਾਂਦੀ ਹੈ । ਇਸ ਨਾਲ ਬਿਜਲੀ ਦੀ ਖ਼ਪਤ ਵਧ ਜਾਂਦੀ ਹੈ ! ਧੁਨੀ ਅਤੇ ਹਵਾ ਪ੍ਰਦੂਸ਼ਣ ਵੀ ਫੈਲਦਾ ਹੈ । ਇਸ ਵਿਚ ਵਰਤੀ ਜਾਣ ਵਾਲੀ ਗੈਸ ਨਾਲ ਓਜ਼ੋਨ ਪਰਤ ਨੂੰ ਨੁਕਸਾਨ ਹੁੰਦਾ ਹੈ ਜਿਸ ਕਰਕੇ ਕੈਂਸਰ ਵਰਗੀਆਂ ਬਿਮਾਰੀਆਂ ਵੱਧ ਰਹੀਆਂ ਹਨ |
![]()
(ੲ) ਛੋਟੇ ਉੱਤਰਾਂ ਵਾਲੇ ਪ੍ਰਨ (Type II)
ਪ੍ਰਸ਼ਨ 1.
ਪੇਂਡੂ ਇਲਾਕਿਆਂ ਵਿਚ ਵਾਤਾਵਰਣ ਨਾਲ ਸੰਬੰਧਿਤ ਸਮੱਸਿਆਵਾਂ ਕਿਹੜੀਆਂ ਹਨ ?
ਉੱਤਰ-
ਪੇਂਡੂ ਇਲਾਕਿਆਂ ਵਿਚ ਵਾਤਾਵਰਣ ਨਾਲ ਸਬੰਧਿਤ ਸਮੱਸਿਆਵਾਂ ਹੇਠ ਲਿਖੇ ਅਨੁਸਾਰ ਹਨ –
- ਜ਼ਿਆਦਾ ਮਾਤਰਾ ਵਿਚ ਕੀਟਨਾਸ਼ਕ ਅਤੇ ਰਸਾਇਣਿਕ ਖਾਦਾਂ ਦੇ ਉਪਯੋਗ ਨਾਲ ਜ਼ਮੀਨ ਦੀ ਉਪਜਾਊ ਸ਼ਕਤੀ ਘੱਟ ਗਈ ਹੈ ।
- ਫ਼ਸਲ ਦਾ ਜ਼ਿਆਦਾ ਝਾੜ ਦੇਣ ਵਾਲੀਆਂ ਕਿਸਮਾਂ ਨੂੰ ਲਗਾਤਾਰ ਉਪਯੋਗ ਵਿਚ ਲਿਆਉਣ ਦੇ ਕਾਰਨ ਭੁਮੀ ਹੇਠਲੇ ਪਾਣੀ ਦਾ ਸਤਰ ਨੀਵਾਂ ਹੋ ਗਿਆ ਹੈ ।
- ਸਿਹਤ ਨਾਲ ਸੰਬੰਧਿਤ ਸਮੱਸਿਆਵਾਂ ਵਿਚ ਵਾਧਾ ਹੋ ਰਿਹਾ ਹੈ ।
- ਲੱਕੜੀ ਅਤੇ ਪਾਥੀਆਂ ਦਾ ਬਾਲਣ ਦੇ ਰੂਪ ਵਿਚ ਉਪਯੋਗ ਕਰਨ ਤੇ ਨਿਕਲਣ ਵਾਲੇ ਧੁੰਏਂ ਦੇ ਕਾਰਨ ਪੇਂਡੂ ਔਰਤਾਂ ਦੀ ਸਿਹਤ ‘ਤੇ ਬੁਰਾ ਪ੍ਰਭਾਵ ਪੈਂਦਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 2.
ਸਾਧਨਾਂ ਦੀ ਸਹੀ ਵੰਡ ਨਾ ਹੋਣ ਕਾਰਨ ਪੈਦਾ ਹੋਣ ਵਾਲੇ ਕੁੱਝ ਵਿਵਾਦਾਂ ਦੀਆਂ ਉਦਾਹਰਨਾਂ ਦਿਉ ।
ਉੱਤਰ-
ਸਾਧਨਾਂ ਦੀ ਸਹੀ ਵੰਡ ਨਾ ਹੋਣ ਕਰਕੇ ਪੈਦਾ ਹੋਣ ਵਾਲੇ ਵਿਵਾਦਾਂ ਵਿੱਚੋਂ ਕੁੱਝ ਵਿਵਾਦ ਹੇਠ ਲਿਖੇ ਹਨ
- ਸਤਲੁਜ-ਯਮੁਨਾ ਲਿੰਕ ਨਹਿਰ ਦੇ ਕਾਰਨ ਪੰਜਾਬ ਅਤੇ ਹਰਿਆਣਾ ਦੇ ਵਿਚਕਾਰ ਵਿਵਾਦ ਹੈ ।
- ਕਾਵੇਰੀ ਨਦੀ ਨੂੰ ਲੈ ਕੇ ਕੇਰਲ ਅਤੇ ਤਾਮਿਲਨਾਡੂ ਦੇ ਵਿਚਕਾਰ ਵਿਵਾਦ ਹੈ !
- ਅੰਤਰਰਾਸ਼ਟਰੀ ਸਤਰ ਤੇ ਮੇਕਾਂਗ ਨਦੀ ਨੂੰ ਲੈ ਕੇ ਵਿਵਾਦ ਹੈ ਜਿਹੜੀ ਚੀਨ, ਮਿਆਂਮਾਰ, ਲਾਓਸ (Laos), ਥਾਈਲੈਂਡ, ਕੰਬੋਡੀਆ ਅਤੇ ਵਿਅਤਨਾਮ ਵਿਚੋਂ ਹੋ ਕੇ ਲੰਘਦੀ ਹੈ ।
ਪ੍ਰਸ਼ਨ 3.
ਸਮੋਗ (Smog) ਕੀ ਹੈ ? ਇਸਦੇ ਬੁਰੇ ਪ੍ਰਭਾਵਾਂ ਦਾ ਵਰਣਨ ਕਰੋ ।
ਉੱਤਰ-
ਘੱਟ ਤਾਪਮਾਨ ਦੇ ਕਾਰਨ ਸਰਦੀਆਂ ਵਿਚ ਉਦਯੋਗਿਕ ਧੂੰਆਂ ਵਾਸ਼ਪਾਂ ਨਾਲ ਮਿਲ ਕੇ ਧੁੰਦ–ਧੁੰਏਂ ਜਾਂ ਸਮੋਗ ਵਿਚ ਬਦਲ ਜਾਂਦਾ ਹੈ । ਇਸ ਨਾਲ ਦਿਖਾਈ ਵੀ ਘੱਟ ਦਿੰਦਾ ਹੈ ਅਤੇ ਇਹ ਅੱਖਾਂ, ਗਲੇ ਅਤੇ ਫੇਫੜੇ ਦੀਆਂ ਬਿਮਾਰੀਆਂ ਦਾ ਕਾਰਨ ਵੀ ਬਣਦਾ ਹੈ । ਧੁੰਦ, ਧੂੰਏਂ ਦੇ ਕਾਰਨ ਹਵਾਈ ਉਡਾਨਾਂ ਅਤੇ ਟ੍ਰੈਫਿਕ ਪ੍ਰਭਾਵਿਤ ਹੁੰਦੇ ਹਨ ।
ਪ੍ਰਸ਼ਨ 4.
ਵਿਸ਼ਵ ਤਾਪਮਾਨ ਵਿਚ ਵਾਧੇ (Global Warming) ਦਾ ਕੀ ਅਰਥ ਹੈ ?
ਉੱਤਰ-
ਵਾਤਾਵਰਣ ਵਿਚ ਕਾਰਬਨ ਡਾਈਆਕਸਾਈਡ ਗੈਸ ਦੀ ਮਾਤਰਾ ਵਧਣ ਨਾਲ ਸ੍ਰੀਨ ਹਾਊਸ ਪ੍ਰਭਾਵ ਪੈਦਾ ਹੁੰਦਾ ਹੈ ਜਿਸਦੇ ਨਾਲ ਪ੍ਰਿਥਵੀ ਦੇ ਤਾਪਮਾਨ ਵਿਚ ਵਾਧਾ ਹੁੰਦਾ ਹੈ । ਇਸ ਤਾਪਮਾਨ ਦੇ ਵਾਧੇ ਨੂੰ ਵਿਸ਼ਵ ਤਾਪਮਾਨ ਵਿਚ ਵਾਧਾ ਕਿਹਾ ਜਾਂਦਾ ਹੈ । ਵਿਸ਼ਵ ਤਾਪਮਾਨ ਦੇ ਵਧਣ ਦੇ ਕਾਰਨ ਧਰੁਵੀ ਬਰਫ਼ ਦੇ ਪਿਘਲਣ ਦੀ ਸੰਭਾਵਨਾ ਹੈ । ਜਿਸਦੇ ਨਾਲ ਦੀਪ ਅਤੇ ਤੱਟੀ ਦੇਸ਼ ਪਾਣੀ ਨਾਲ ਭਰ ਜਾਣਗੇ ।
ਪ੍ਰਸ਼ਨ 5.
ਗੰਦੀਆਂ ਬਸਤੀਆਂ (Slums/Slum areas) ਦੇ ਵਿਕਾਸ ਦੇ ਕਾਰਨ ਦੱਸੋ !
ਉੱਤਰ-
ਗ਼ਰੀਬ ਪੇਂਡੂ ਲੋਕ ਆਪਣੀ ਰੋਜ਼ੀ-ਰੋਟੀ ਕਮਾਉਣ ਲਈ ਸ਼ਹਿਰੀ ਇਲਾਕਿਆਂ ਵਲ ਜਾਂਦੇ ਹਨ । ਇਸਦੇ ਨਾਲ ਸ਼ਹਿਰਾਂ ਵਿਚ ਰਹਿਣ-ਸਹਿਣ ਸੰਬੰਧੀ ਸਮੱਸਿਆਵਾਂ ਪੈਦਾ ਹੁੰਦੀਆਂ ਹਨ । ਨਿਵਾਸ ਸਥਾਨਾਂ ਦੀ ਕਮੀ ਦੇ ਕਾਰਨ ਪੇਂਡੂ ਲੋਕ ਸ਼ਹਿਰ ਦੇ ਬਾਹਰੀ ਇਲਾਕਿਆਂ ਵਿਚ ਖ਼ਾਲੀ ਭੁਮੀ ਤੇ ਝੁੱਗੀਆਂ-ਝੌਪੜੀਆਂ ਬਣਾ ਲੈਂਦੇ ਹਨ । ਇਹੀ ਝੁੱਗੀਆਂ-ਝੌਪੜੀਆਂ ਗੰਦੀਆਂ ਬਸਤੀਆਂ ਨੂੰ ਜਨਮ ਦਿੰਦੀਆਂ ਹਨ । ਇਨ੍ਹਾਂ ਬਸਤੀਆਂ ਵਿਚ ਜੀਵਨ ਸਤਰ ਨੀਵੇਂ ਦਰਜੇ ਦਾ ਹੁੰਦਾ ਹੈ ਅਤੇ ਪਾਣੀ ਦਾ ਪ੍ਰਯੋਗ ਵੀ ਖੁੱਲੇ ਸਥਾਨਾਂ ‘ਤੇ ਹੁੰਦਾ ਹੈ । ਇਨ੍ਹਾਂ ਬਸਤੀਆਂ ਦੇ ਕਾਰਨ ਹੀ ਕਈ ਵਾਤਾਵਰਣੀ ਸਮੱਸਿਆਵਾਂ ਪੈਦਾ ਹੁੰਦੀਆਂ ਹਨ |
ਪ੍ਰਸ਼ਨ 6.
ਭੂਮੀ ਸਾਧਨ (Land Resources) ਅੱਜ ਕਿਸ ਪ੍ਰਕਾਰ ਖ਼ਤਰੇ ਵਿਚ ਹਨ ?
ਉੱਤਰ-
ਭੂਮੀ ਇਕ ਬੁਨਿਆਦੀ ਸਾਧਨ ਹੈ । ਇਹ ਇਕ ਸਥਿਰ ਸਾਧਨ ਹੈ ਇਸ ਲਈ ਇਸਦਾ ਉਪਯੋਗ ਸਹੀ ਢੰਗ ਨਾਲ ਕਰਨਾ ਚਾਹੀਦਾ ਹੈ ਕਿਉਂਕਿ ਦੇਸ਼ ਵਿਚ ਉਪਲੱਬਧ ਭੂਮੀ ਦੀ ਇਕ ਸੀਮਾ ਹੈ | ਪਰੰਤੂ ਅੱਜ ਵਸੋਂ ਦੇ ਵਧਣ ਨਾਲ ਅਤੇ ਜ਼ਰੂਰਤ ਤੋਂ ਜ਼ਿਆਦਾ ਵਸਤੁਆਂ ਦੇ ਉਪਭੋਗ ਦੇ ਕਾਰਨ ਭੂਮੀ ਦਾ ਬਹੁਤ ਜ਼ਿਆਦਾ ਸ਼ੋਸ਼ਣ ਕੀਤਾ ਜਾ ਰਿਹਾ ਹੈ । ਇਸ ਨਾਲ ਭੂਮੀ ‘ਤੇ ਦਬਾਉ ਵਧ ਰਿਹਾ ਹੈ ਅਤੇ ਸਾਡੇ ਉਪਯੋਗੀ ਸਾਧਨ ਵੀ ਖ਼ਤਰੇ ਵਿਚ ਹਨ | ਗਲਤ ਢੰਗ ਨਾਲ ਖੇਤੀ ਕਰਨ ਨਾਲ ਧਰਤੀ ਦੀ ਉਪਜਾਊ ਸ਼ਕਤੀ ਵੀ ਘੱਟ ਰਹੀ ਹੈ । ਮਿੱਟੀ ਵਿਚਲੇ ਸੂਖਮ ਜੀਵ ਵੀ ਨਸ਼ਟ ਹੋ ਰਹੇ ਹਨ । ਇਸ ਤਰ੍ਹਾਂ ਭੂਮੀ ਸਾਧਨਾਂ ਨੂੰ ਗੰਭੀਰ ਵਾਤਾਵਰਣੀ ਖ਼ਤਰਿਆਂ ਦਾ ਸਾਹਮਣਾ ਕਰਨਾ ਪੈ ਰਿਹਾ ਹੈ ।
![]()
ਪ੍ਰਸ਼ਨ 7.
ਪਾਣੀ ਦਾ ਸਹੀ ਨਿਕਾਸ ਨਾ ਹੋਣ ਦੇ ਕਾਰਨ ਪੈਦਾ ਹੋਈਆਂ ਸਮੱਸਿਆਵਾਂ ਦੀ ਜਾਣਕਾਰੀ ਦਿਉ ।
ਉੱਤਰ-
ਪਾਣੀ ਦਾ ਸਹੀ ਨਿਕਾਸ ਨਾ ਹੋਣ ਦੇ ਕਾਰਨ ਕਈ ਪ੍ਰਕਾਰ ਦੀਆਂ ਵਾਤਾਵਰਣਿਕ ਸਮੱਸਿਆਵਾਂ ਪੈਦਾ ਹੁੰਦੀਆਂ ਹਨ । ਵਿਅਰਥ ਕੂੜੇ ਦੇ ਢੇਰ ਵਿਚ ਵਾਧਾ ਹੋਣ ਦੇ ਕਾਰਨ ਪਾਣੀ ਪ੍ਰਦੂਸ਼ਣ ਵਧ ਜਾਂਦਾ ਹੈ । ਪ੍ਰਦੂਸ਼ਿਤ ਪਾਣੀ ਦੇ ਉਪਯੋਗ ਦੇ ਨਾਲ ਕਈ ਪ੍ਰਕਾਰ ਦੀਆਂ ਬਿਮਾਰੀਆਂ ਪੈਦਾ ਹੋ ਜਾਂਦੀਆਂ ਹਨ । ਸੀਵਰੇਜ ਪਾਈਪਾਂ ਦੇ ਲੀਕ ਹੋਣ ਨਾਲ ਪਾਣੀ ਵਿਚ ਪਾਏ ਜਾਣ ਵਾਲੇ ਜੀਵ-ਜੰਤੂ ਵੀ ਪ੍ਰਭਾਵਿਤ ਹੁੰਦੇ ਹਨ ।
ਪ੍ਰਸ਼ਨ 8.
ਪਾਣੀ ਦੇ ਜ਼ਿਆਦਾ ਉਪਯੋਗ ਹੋਣ ਦੇ ਕਾਰਨ ਕੀ ਸਮੱਸਿਆਵਾਂ ਪੈਦਾ ਹੋ ਰਹੀਆਂ ਹਨ ?
ਉੱਤਰ-
ਪਾਣੀ ਦੇ ਜ਼ਿਆਦਾ ਉਪਯੋਗ ਹੋਣ ਦੇ ਕਾਰਨ ਹੇਠਾਂ ਲਿਖੀਆਂ ਸਮੱਸਿਆਵਾਂ ਪੈਦਾ ਹੋ ਰਹੀਆਂ ਹਨ –
- ਸ਼ੁੱਧ ਪਾਣੀ ਭੰਡਾਰਾਂ ਵਿਚ ਪਾਣੀ ਘੱਟ ਰਿਹਾ ਹੈ ।
- ਭੂਮੀਗਤ ਪਾਣੀ ਦਾ ਸਤਰ ਘੱਟ ਰਿਹਾ ਹੈ ।
- ਪੀਣ ਵਾਲੇ ਪਾਣੀ ਦਾ ਸੰਕਟ ਪੈਦਾ ਹੋ ਰਿਹਾ ਹੈ ।
ਪ੍ਰਸ਼ਨ 9.
ਸਵਾਸਥ (ਸਿਹਤ ਦੀਆਂ ਸੇਵਾਵਾਂ ਵਿਚ ਵਧ ਰਹੇ ਦਬਾਅ ਉੱਤੇ ਟਿੱਪਣੀ ਕਰੋ ।
ਉੱਤਰ-
ਵਾਤਾਵਰਣ ਪ੍ਰਦੂਸ਼ਣ ਜਾਂ ਵਧ ਰਹੀਆਂ ਬੀਮਾਰੀਆਂ ਦੇ ਕਾਰਨ ਸਿਹਤ ਸੇਵਾਵਾਂ ਉੱਤੇ ਦਬਾਅ ਵਧ ਰਿਹਾ ਹੈ । ਸਿਹਤ ਸੇਵਾਵਾਂ ਅਤੇ ਆਰੋਗ ਸੁਵਿਧਾਵਾਂ ਦੀ ਕਮੀ ਦੇ ਕਾਰਨ ਕਈ ਬੀਮਾਰੀਆਂ ਫੈਲ ਰਹੀਆਂ ਹਨ । ਹਸਪਤਾਲਾਂ ਵਿਚ ਮਰੀਜ਼ਾਂ ਦੀ ਸੰਖਿਆ ਵਿਚ ਵਾਧਾ ਹੋ ਰਿਹਾ ਹੈ। ਇਸਦੇ ਕਾਰਨ ਦਵਾਈਆਂ, ਯੰਤਰਾਂ ਅਤੇ ਬਿਸਤਰਿਆਂ ਦੀ ਕਮੀ ਹੋ ਰਹੀ ਹੈ । ਸਿਹਤ ਸੇਵਾਵਾਂ ‘ਤੇ ਵਧ ਰਹੇ ਦਬਾਅ ਨੂੰ ਘੱਟ ਕਰਨ ਦੇ ਲਈ ਰੋਗ ਪੈਦਾ ਕਰਨ ਵਾਲੀ ਸਥਿਤੀ ਨੂੰ ਬਦਲਣਾ ਪਏਗਾ । ਇਸਦੇ ਲਈ ਜ਼ਰੂਰੀ ਹੈ ਕਿ ਵਾਤਾਵਰਣ ਰਾਹੀਂ ਫੈਲਦੀਆਂ ਬਿਮਾਰੀਆਂ ਦਾ ਅੰਤ ਕੀਤਾ ਜਾਵੇ ।
ਪ੍ਰਸ਼ਨ 10.
ਸ਼ਹਿਰੀਕਰਨ ਦੇ ਮਾੜੇ ਪ੍ਰਭਾਵਾਂ ਨੂੰ ਰੋਕਣ ਲਈ ਕੀ-ਕੀ ਕੀਤਾ ਜਾ ਸਕਦਾ ਹੈ ?
ਉੱਤਰ-
ਸ਼ਹਿਰੀਕਰਨ ਦੇ ਮਾੜੇ ਪ੍ਰਭਾਵਾਂ ਨੂੰ ਘੱਟ ਕਰਨ ਲਈ ਵਧੀਆ ਯੋਜਨਾ ਦਾ ਨਿਰਮਾਣ ਕਰਨਾ ਬਹੁਤ ਜ਼ਰੂਰੀ ਹੈ । ਇਸ ਲਈ ਇਸ ਯੋਜਨਾ ਵਿਚ ਹੇਠ ਲਿਖੀਆਂ ਗਤੀਵਿਧੀਆਂ ਦਾ ਹੋਣਾ ਜ਼ਰੂਰੀ ਹੈ –
- ਸ਼ਹਿਰਾਂ ਦੇ ਚਾਰੇ ਪਾਸੇ ਰਿਹਾਇਸ਼ੀ ਕਲੋਨੀਆਂ ਦਾ ਵਿਕਾਸ ਕਰਨ ਵੇਲੇ, ਉਹਨਾਂ ਵਿਚ ਚੌੜੀਆਂ ਹਰੀਆਂ ਪੱਟੀਆਂ ਰੱਖਣਾ; ਜਿਵੇਂ ਇੰਗਲੈਂਡ ਵਿਚ ਹੈ ।
- ਗਰੀਬ ਲੋਕਾਂ ਦਾ ਇਲਾਜ ਕਰਨਾ ।
- ਪਦਾਰਥਾਂ ਦਾ ਪੁਨਰ ਨਿਰਮਾਣ ਕਰਨਾ ।
- ਮੁੱਖ ਸ਼ਹਿਰ ਦੇ ਆਸ-ਪਾਸ ਗੰਦੀਆਂ ਬਸਤੀਆਂ ਵਸਾਉਣ ਦੀ ਮਨਾਹੀ ।
ਪ੍ਰਸ਼ਨ 11.
ਕੀਟਨਾਸ਼ਕਾਂ (Insecticides) ਦੀ ਜ਼ਿਆਦਾ ਵਰਤੋਂ ਕਰਨ ਨਾਲ ਕੀ-ਕੀ ਸਮੱਸਿਆਵਾਂ ਉਤਪੰਨ ਹੁੰਦੀਆਂ ਹਨ ?
ਉੱਤਰ-
ਆਬਾਦੀ ਦੇ ਵਾਧੇ ਦੇ ਕਾਰਨ ਖਾਣ ਵਾਲੇ ਪਦਾਰਥਾਂ ਦੀ ਵਧਦੀ ਮੰਗ ਨੂੰ ਪੂਰਾ ਕਰਨ ਦੇ ਲਈ ਖੇਤੀ ਯੋਗ ਭੂਮੀ ਨੂੰ ਪਾਣੀ ਦੇ ਸਾਧਨਾਂ ਅਤੇ ਕੀਟਨਾਸ਼ਕ ਦਵਾਈਆਂ, ਰਸਾਇਣਿਕ ਖਾਦਾਂ ਆਦਿ ਦੀ ਸਹਾਇਤਾ ਨਾਲ ਜ਼ਿਆਦਾ ਉਪਜਾਊ ਬਣਾਉਣ ਦੇ ਯਤਨ ਕੀਤੇ ਜਾ ਰਹੇ ਹਨ । ਇਸ ਨਾਲ ਖਾਣ ਵਾਲੇ ਪਦਾਰਥਾਂ ਦੀ ਉਤਪਾਦਕਤਾ 50% ਤੋਂ ਜ਼ਿਆਦਾ ਵਧ ਗਈ ਹੈ ਪਰ ਰਸਾਇਣਿਕ ਖਾਦਾਂ ਅਤੇ ਕੀਟਨਾਸ਼ਕਾਂ ਦੀ ਵਰਤੋਂ ਨਾਲ ਬਹੁਤ ਸਾਰੀਆਂ ਸਮੱਸਿਆਵਾਂ ਪੈਦਾ ਹੋ ਗਈਆਂ ਹਨ ।
ਜ਼ਿਆਦਾਤਰ ਕੀਟਨਾਸ਼ਕ ਦਵਾਈਆਂ ਮਨੁੱਖ ਤਕ ਪਹੁੰਚਦੇ-ਪਹੁੰਚਦੇ ਆਪਣੀ ਮਾਤਰਾ ਵਿਚ ਜੈਵ-ਅਵਰਧਨ ਜਾਂ ਜੀਵ ਵਿਸ਼ਾਲੀਕਰਨ (Bio magnification) ਰਾਹੀਂ ਵਾਧਾ ਕਰ ਲੈਂਦੀਆਂ ਹਨ । ਜਿਸ ਨਾਲ ਇਹ ਵਿਸ਼ੈਲੀਆਂ ਹੋ ਜਾਂਦੀਆਂ ਹਨ । ਇਹਨਾਂ ਦਾ ਜ਼ਿਆਦਾ ਉਪਯੋਗ ਸਜੀਵ ਪ੍ਰਾਣੀਆਂ ਦੀਆਂ ਮੌਤਾਂ ਦਾ ਕਾਰਨ ਬਣਦਾ ਹੈ । ਭੋਜਨ ਲੜੀ ਵਿਚ ਜੈਵ-ਅਵਰਧਨ ਨਾਲ ਕਈ ਬੀਮਾਰੀਆਂ ਫੈਲਦੀਆਂ ਹਨ | ਖੇਤਾਂ ਦੇ ਉੱਪਰੀ ਪਾਣੀ ਵਹਾਅ ਦੇ ਦੁਆਰਾ ਪਾਣੀ ਵਿਚ ਨਾਈਟਰੋਜਨ ਦਾ ਪ੍ਰਦੂਸ਼ਣ ਵਧਦਾ ਹੈ । ਜਿਸਦੇ ਕਾਰਨ ਬੱਚਿਆਂ ਨੂੰ ਸਿਆਨੋਸਿਸ ਦੀ ਬਿਮਾਰੀ ਹੁੰਦੀ ਹੈ । ਜਿਸਦੇ ਇਲਾਵਾ ਜਲੀ ਜੀਵ-ਜੰਤੂ ਵੀ ਇਹਨਾਂ ਨਾਲ ਪ੍ਰਭਾਵਿਤ ਹੁੰਦੇ ਹਨ ।
ਪ੍ਰਸ਼ਨ 12.
ਰਹਿੰਦ-ਖੂੰਹਦ ਦਾ ਗ਼ਲਤ ਪ੍ਰਬੰਧ ਕਿਸ ਤਰ੍ਹਾਂ ਨਾਲ ਵਾਤਾਵਰਣ ਦੀ ਸਮੱਸਿਆ ਬਣਦਾ ਹੈ ?
ਉੱਤਰ-
ਉਹ ਅਣਇੱਛਤ ਘਰੇਲੂ ਜਾਂ ਉਦਯੋਗਿਕ ਠੋਸ ਪਦਾਰਥ ਜਿਨ੍ਹਾਂ ਨੂੰ ਕੂੜੇ ਦੇ ਰੂਪ ਵਿਚ ਸੁੱਟ ਦਿੱਤਾ ਜਾਂਦਾ ਹੈ ਰਹਿੰਦ-ਖੂੰਹਦ ਕਹਾਉਂਦੇ ਹਨ | ਸ਼ਹਿਰਾਂ ਵਿਚ ਰਹਿੰਦ-ਖੂੰਹਦ ਦਾ ਪ੍ਰਬੰਧ ਇਕ ਬਹੁਤ ਵੱਡੀ ਸਮੱਸਿਆ ਹੈ । ਵਸੋਂ ਦੇ ਜ਼ਿਆਦਾ ਹੋਣ ਦੇ ਕਾਰਨ ਸ਼ਹਿਰਾਂ ਵਿਚ ਰਹਿੰਦ-ਖੂੰਹਦ ਦੇ ਉਤਪਾਦਨ ਦੀ ਮਾਤਰਾ ਵੀ ਵਧ ਹੈ । ਠੋਸ ਰਹਿੰਦ-ਖੂੰਹਦ ਦੇ ਮੁੱਖ ਸ੍ਰੋਤ ਘਰੇਲੂ ਪਦਾਰਥ, ਉਦਯੋਗਿਕ ਪਦਾਰਥ ਅਤੇ ਹਸਪਤਾਲਾਂ ਦੇ ਰਹਿੰਦ-ਖੂੰਹਦ ਹਨ । ਰਹਿੰਦਖੂੰਹਦ ਪਦਾਰਥਾਂ ਦੇ ਗ਼ਲਤ ਪ੍ਰਬੰਧ ਦੇ ਸਿੱਟੇ ਵਜੋਂ ਸ਼ਹਿਰਾਂ ਵਿਚ ਕੂੜੇ ਦੇ ਢੇਰ ਲੱਗੇ ਹੋਏ ਹਨ ।
ਜਿਨ੍ਹਾਂ ਉੱਪਰ ਬੀਮਾਰੀ ਦੇ ਕੀਟਾਣੂ ਪੈਦਾ ਹੁੰਦੇ ਹਨ ਅਤੇ ਹੈਜਾ, ਪੇਚਿਸ਼ ਆਦਿ ਰੋਗਾਂ ਦੀ ਸੰਭਾਵਨਾ ਵਧ ਜਾਂਦੀ ਹੈ । ਠੋਸ ਰਹਿੰਦ-ਖੂੰਹਦ ਦੇ ਅਪਘਟਨ ਨਾਲ ਓਜ਼ੋਨ ਅਤੇ ਹੋਰ ਜ਼ਹਿਰੀਲੀਆਂ ਗੈਸਾਂ ਬਣਦੀਆਂ ਹਨ ਜੋ ਹਵਾ ਪ੍ਰਦੂਸ਼ਣ ਫੈਲਾਉਂਦੀਆਂ ਹਨ | ਪਲਾਸਟਿਕ ਦੀਆਂ ਥੈਲੀਆਂ ਨਾਲ ਭੂਮੀ ਪ੍ਰਦੂਸ਼ਣ ਹੁੰਦਾ ਹੈ । ਇਸ ਪ੍ਰਕਾਰ ਰਹਿੰਦ-ਖੂੰਹਦ ਪ੍ਰਬੰਧਨ ਸ਼ਹਿਰਾਂ ਦੀ ਵਾਤਾਵਰਣ ਦੀ ਗੰਭੀਰ ਸਮੱਸਿਆ ਹੈ ।
(ਸ) ਵੱਡੇ ਉੱਤਰਾਂ ਵਾਲੇ ਪ੍ਰਸ਼ਨ –
ਪ੍ਰਸ਼ਨ 1.
ਕੁਦਰਤੀ ਸਾਧਨਾਂ ਦੀ ਬੇਲੋੜੀ ਖਪਤ ਅਤੇ ਦੋਹਣ ਉੱਤੇ ਸੰਖੇਪ ਟਿੱਪਣੀ ਕਰੋ ।
ਉੱਤਰ-
ਕੁਦਰਤੀ ਸਾਧਨਾਂ ਤੋਂ ਮਤਲਬ ਮਨੁੱਖ ਨੂੰ ਉਪਲੱਬਧ ਪ੍ਰਕ੍ਰਿਤੀ ਦੇ ਉਨ੍ਹਾਂ ਭੰਡਾਰਾਂ ਤੋਂ ਹੈ ਜੋ ਮਨੁੱਖ ਦੀਆਂ ਜ਼ਰੂਰਤਾਂ ਦੀ ਪੂਰਤੀ ਕਰਦੇ ਹਨ |ਇਹਨਾਂ ਸਾਧਨਾਂ ਵਿਚ ਵਣ, ਜਲ, ਖਣਿਜ ਆਦਿ ਸ਼ਾਮਿਲ ਹਨ । ਵਧਦੀ ਹੋਈ ਵਸੋਂ ਅਤੇ ਸੁੱਖ-ਸਹੂਲਤਾਂ ਦੀ ਲਾਲਸਾ ਦੇ ਕਾਰਨ ਮਨੁੱਖ ਨੇ ਇਨ੍ਹਾਂ ਸਾਧਨਾਂ ਦਾ ਵੱਧ ਪਤਨ ਕੀਤਾ ਹੈ । ਜਿਸ ਦੇ ਸਿੱਟੇ ਵਜੋਂ ਇਨ੍ਹਾਂ ਦਾ ਵਿਘਟਨ ਹੋ ਰਿਹਾ ਹੈ। ਜ਼ਿਆਦਾ ਵਰਤੋਂ ਦਾ ਭਿੰਨ-ਭਿੰਨ ਪ੍ਰਾਕ੍ਰਿਤਕ ਸਾਧਨਾਂ ਉੱਤੇ ਪ੍ਰਤੀਕੂਲ ਪ੍ਰਭਾਵ ਪੈਂਦਾ ਹੈ ਜਿਸਦਾ ਵਰਣਨ ਹੇਠਾਂ ਲਿਖੇ ਅਨੁਸਾਰ ਕੀਤਾ ਜਾ ਸਕਦਾ ਹੈ
- ਵਣ · ਸੰਸਾਧਨ (Forests resources)-ਵਣ ਸਾਧਨ ਬਹੁਤ ਲਾਭਕਾਰੀ ਸਾਧਨ ਹਨ |ਵਣ ਪਰਿਸਥਿਤੀ ਸੰਤੁਲਨ ਨੂੰ ਬਣਾਈ ਰੱਖਣ ਦੇ ਲਈ ਮਹੱਤਵਪੂਰਨ ਭੂਮਿਕਾ ਨਿਭਾਉਂਦੀ ਹੈ | ਵਣ ਪ੍ਰਦੂਸ਼ਿਤ ਹਵਾ ਨੂੰ ਸਾਫ ਕਰਨ ਲਈ ਸਹਾਇਕ ਹਨ | ਵਣ ਆਕਸੀਜਨ ਦਾ ਮੁੱਖ ਸੋਤ ਹੈ । ਵਣਾਂ ਤੋਂ ਸਾਨੂੰ ਇਮਾਰਤੀ ਲੱਕੜੀ, ਬਾਲਣ ਦੀ ਲੱਕੜੀ ਅਤੇ ਹੋਰ ਉਪਯੋਗੀ ਪਦਾਰਥ ਉਪਲੱਬਧ ਹੁੰਦੇ ਹਨ । ਇਸਦੇ ਇਲਾਵਾ ਵਣ-ਪਾਣੀ ਚੱਕਰ ਉੱਤੇ ਮਹੱਤਵਪੂਰਨ ਪ੍ਰਭਾਵ ਪਾਉਂਦੇ ਹਨ ਅਤੇ ਭੂਮੀ-ਖੋਰਨ ਅਤੇ ਮਿੱਟੀ ਦੇ ਪ੍ਰਦੂਸ਼ਣ ਨੂੰ ਰੋਕਦੇ ਹਨ ਪਰ ਵਰਤਮਾਨ ਸਮੇਂ ਵਿਚ ਮਨੁੱਖੀ ਕਿਰਿਆਵਾਂ ਵਣਾਂ ਦੇ ਵਿਨਾਸ਼ ਦਾ ਪ੍ਰਮੁੱਖ ਕਾਰਨ ਬਣ ਰਹੀਆਂ ਹਨ । ਇਨ੍ਹਾਂ ਮਨੁੱਖੀ ਕਿਰਿਆਵਾਂ ਦੇ ਕਾਰਨ ਵਣਾਂ ਤੋਂ ਉਪਲੱਬਧ ਸਾਧਨਾਂ ਤੋਂ ਸਾਡੀਆਂ ਆਉਣ ਵਾਲੀਆਂ ਪ੍ਰਭਾਵ ਪੀੜ੍ਹੀਆਂ ਵਾਂਝੀਆਂ ਰਹਿ ਜਾਣਗੀਆਂ।
ਇਨ੍ਹਾਂ ਮਨੁੱਖੀ ਕਿਰਿਆਵਾਂ ਵਿਚ ਪ੍ਰਮੁੱਖ ਤੌਰ ਤੇ ਹੇਠ ਲਿਖੇ ਕਾਰਨ ਉੱਤਰਦਾਈ ਹਨ –
- ਖੇਤੀ ਭੂਮੀ ਦਾ ਵਿਸਤਾਰ
- ਬਦਲਵੀਂ ਖੇਤੀ
- ਬੰਨ/ਡੈਮ ਯੋਜਨਾਵਾਂ
- ਖਾਧ ਪ੍ਰਕਿਰਿਆਵਾਂ
- ਵਪਾਰਿਕ ਗਤੀਵਿਧੀਆਂ
- ਵਿਕਾਸ ਗਤੀਵਿਧੀਆਂ
- ਬਾਲਣ ਤੇ ਚਾਰਨ ਲਈ
- ਸੜਕ ਤੇ ਰੇਲ ਮਾਰਗਾਂ ਦਾ ਵਿਕਾਸ
- ਖਰਾਬ ਵਣ ਪ੍ਰਬੰਧ ।
ਵਣ ਵਿਨਾਸ਼ ਸਾਰੇ ਵਾਤਾਵਰਣ ਨੂੰ ਪ੍ਰਭਾਵਿਤ ਕਰਦਾ ਹੈ । ਇਸਦੇ ਵਿਨਾਸ਼ ਦੇ ਕਾਰਨ ਜੰਗਲੀ ਜੀਵਾਂ ਵਿਚ ਕਮੀ ਹੋ ਰਹੀ ਹੈ, ਤਾਪਮਾਨ ਦੇ ਜ਼ਿਆਦਾ ਹੋਣ ਦੀ ਸਮੱਸਿਆ ਵਧ ਰਹੀ ਹੈ ਅਤੇ ਵਾਯੂਮੰਡਲ ਦੀ ਪ੍ਰਦੂਸ਼ਿਕਤਾ ਵਧ ਰਹੀ ਹੈ । ਇਸਦੇ ਇਲਾਵਾ ਭੁਮੀ ਕਮਜ਼ੋਰ ਹੋ ਕੇ ਬੰਜਰ ਹੋ ਰਹੀ ਹੈ ਅਤੇ ਵਾਤਾਵਰਣ ਦੇ ਖ਼ਰਾਬ ਹੋਣ ਦੀਆਂ ਪਰਿਸਥਿਤੀਆਂ ਉਤਪੰਨ ਹੋ ਰਹੀਆਂ ਹਨ |
2. ਪਾਣੀ ਸੰਸਾਧਨ (Water resources)-ਪਾਣੀ ਕੁਦਰਤ ਦਾ ਵਡਮੁੱਲਾ ਉਪਹਾਰ ਹੈ । ਇਹ ਜੀਵ ਮੰਡਲ ਦਾ ਆਧਾਰ ਹੈ । ਪਾਣੀ ਪ੍ਰਿਥਵੀ ਉੱਤੇ ਸਾਰੇ ਪ੍ਰਕਾਰ ਦੇ ਜੀਵਨ ਨੂੰ ਅਸਤਿੱਤਵ ਵਿਚ ਰੱਖਣ ਦੇ ਲਈ ਜ਼ਰੂਰੀ ਹੈ । ਸਭ ਮਨੁੱਖੀ ਕਿਰਿਆਵਾਂ ਅਤੇ ਆਰਥਿਕ ਵਿਕਾਸ ਪ੍ਰਤੱਖ ਅਤੇ ਅਪ੍ਰਤੱਖ ਰੂਪ ਨਾਲ ਪਾਣੀ ‘ਤੇ ਹੀ ਨਿਰਭਰ ਕਰਦੇ ਹਨ । ਅੱਜ ਦਾ ਮਨੁੱਖ ਪਾਣੀ ਦਾ ਪ੍ਰਯੋਗ ਨਹਾਉਣ ਲਈ, ਕੱਪੜੇ ਧੋਣ ਲਈ, ਸਾਫ-ਸਫਾਈ ਤੋਂ ਲੈ ਕੇ ਸਿੰਜਾਈ ਅਤੇ ਬਿਜਲੀ ਉਤਪਾਦਨ ਤਕ ਦੇ ਲਈ ਕਰਦਾ ਹੈ । ਇਸ ਲਈ ਇਹ ਵਰਤੋਂ ਬਹੁਤ ਖਤਰਨਾਕ ਰੂਪ ਧਾਰਨ ਕਰ ਚੁੱਕੀ ਹੈ । ਇਕ ਅਨੁਮਾਨ ਦੇ ਅਨੁਸਾਰ ਪੂਰੇ ਸੰਸਾਰ ਵਿਚ ਖੇਤੀ, ਉਦਯੋਗਾਂ ਅਤੇ ਘਰੇਲੂ ਉਪਯੋਗਾਂ ਦੇ ਲਈ ਪਾਣੀ ਦੀ ਵਰਤੋਂ 65%, 25, 5% ਤਕ ਕੀਤੀ ਜਾਂਦੀ ਹੈ ।
ਵਜੋਂ ਵਿਸਫੋਟ ਦੇ ਜ਼ਿਆਦਾ ਵਿਸਤਾਰ ਨੂੰ ਪਾਣੀ ਮੁਹੱਇਆ ਕਰਾਉਣ ਦੇ ਲਈ ਵੱਡੇ ਬੰਨ੍ਹਾਂ ਅਤੇ ਜਲ-ਸਾਧਨਾਂ ਦਾ ਜਾਲ ਵਿਛਾਣਾ ਪੈਂਦਾ ਹੈ । ਇਸ ਨਾਲ ਪੂਰੇ ਸਾਲ ਤਕ ਪਾਣੀ ਪੂਰਤੀ ਨਿਯੰਤਰਿਤ ਰਹਿੰਦੀ ਹੈ | ਪਰ ਇਸ ਤਰ੍ਹਾਂ ਦੇ ਪਾਣੀ ਦੇ ਜ਼ਿਆਦਾ ਵੱਡੇ ਖੇਤਰ ਵਿਚ ਵਾਸ਼ਪੀਕਰਨ ਦੁਆਰਾ ਇਸਦੀ ਹਾਨੀ ਹੋਣ ਦੇ ਕਾਰਨ, ਇਹ ਪੁਰਨ ਵਹਾਅ ਨੂੰ ਘੱਟ ਕਰ ਦਿੰਦਾ ਹੈ । ਇਸਦੇ ਇਲਾਵਾ ਭੂਮੀਗਤ ਪਾਣੀ ਪੱਧਰ ਡਿੱਗ ਰਿਹਾ ਹੈ ਅਤੇ ਬਨਸਪਤੀ ਅਤੇ ਰਹਿਣ ਪਰਿਸਥਿਤੀਆਂ ਤੇ ਉਲਟ ਪ੍ਰਭਾਵ ਪੈ ਰਿਹਾ ਹੈ ।
3. ਖਣਿਜੀ ਸੰਸਾਧਨ (Minerals resources)-ਖਣਿਜੀ ਸਾਧਨ ਆਰਥਿਕ ਅਤੇ ਉਦਯੋਗਿਕ ਵਿਕਾਸ ਨੂੰ ਆਧਾਰ ਪ੍ਰਦਾਨ ਕਰਦੇ ਹਨ | ਖਣਿਜੀ ਸਾਧਨਾਂ ਦੇ ਸੋਤ ਸੀਮਿਤ ਹਨ ।ਇਸਦਾ ਅਰਥ ਹੈ ਕਿ ਨੇੜੇ ਦੇ ਭਵਿੱਖ ਵਿਚ ਇਹਨਾਂ ਦੀ ਜ਼ਿਆਦਾ ਵਰਤੋਂ ਦੇ ਸਿੱਟੇ ਵਜੋਂ ਖਣਿਜਾਂ ਦੀ ਘਾਟ ਦਾ ਸੰਕਟ ਪੈਦਾ ਹੋ ਸਕਦਾ ਹੈ । ਵਧਦੀ ਹੋਈ ਆਬਾਦੀ, ਉਦਯੋਗੀਕਰਨ ਆਦਿ ਦੇ ਕਾਰਨ ਇਨ੍ਹਾਂ ਦੀ ਮੰਗ ਵਿਚ ਵਾਧਾ ਹੋਇਆ ਹੈ । ਇਹਨਾਂ ਦੀ ਪੂਰਤੀ ਦੇ ਲਈ ਖਣਿਜੀ ਸਾਧਨਾਂ ਦਾ ਵਧ ਦੋਹਣ ਹੋ ਰਿਹਾ ਹੈ । ਬਿਨਾਂ ਸੋਚੇ ਸਮਝੇ ਇਹਨਾਂ ਦਾ ਖਾਤਮਾ ਕੀਤਾ ਜਾ ਰਿਹਾ ਹੈ ।
ਇਹਨਾਂ ਢੰਗਾਂ ਦੇ ਸਿੱਟੇ ਵਜੋਂ ਵਾਤਾਵਰਣ ਸੰਕਟ ਵਧਦਾ ਜਾ ਰਿਹਾ ਹੈ । ਜ਼ਿਆਦਾ ਉਪਯੋਗ ਦੇ ਗ਼ਲਤ ਤਰੀਕਿਆਂ ਦੇ ਕਾਰਨ ਵਣ ਵਿਨਾਸ਼, ਭੋਂ-ਖੋਰ, ਧਰਾਤਲੀ ਅਤੇ ਭੂਮੀਗਤ ਪਾਣੀ ਪ੍ਰਦੂਸ਼ਣ, ਹਵਾ ਪ੍ਰਦੂਸ਼ਣ ਅਤੇ ਜੰਗਲੀ ਜੀਵਾਂ ਦਾ ਖ਼ਾਤਮਾ ਹੋ ਰਿਹਾ ਹੈ । ਇਸ ਤਰ੍ਹਾਂ ਕਰਨ ਨਾਲ ਲੋਕਾਂ ਦੀ ਸਿਹਤ ‘ਤੇ ਵੀ ਬੁਰਾ ਪ੍ਰਭਾਵ ਪੈਂਦਾ ਹੈ । ਖਣਿਜੀ ਸਾਧਨਾਂ ਦੇ ਅਤਿ ਦੋਹਣ ਨਾਲ ਬਹੁਤ ਸਾਰੀਆਂ ਗੰਭੀਰ ਸਮੱਸਿਆਵਾਂ ਜਨਮ ਲੈ ਰਹੀਆਂ ਹਨ ।
![]()
4. ਭੂਮੀ ਸੰਸਾਧਨ (Land Resources)-ਭੂਮੀ ਇਕ ਅਧਾਰਭੂਤ ਸੰਸਾਧਨ ਹੈ । ਇਹ ਇਕ ਸਥਿਰ ਸੰਸਾਧਨ ਹੈ । ਇਸ ਲਈ ਇਹਨਾਂ ਦਾ ਪ੍ਰਯੋਗ ਸੰਤੁਲਿਤ ਤਰੀਕੇ ਨਾਲ ਕਰਨਾ ਚਾਹੀਦਾ ਹੈ ਕਿਉਂਕਿ ਦੇਸ਼ ਵਿਚ ਉਪਲੱਬਧ ਭੂਮੀ ਦੀ ਇਕ ਸੀਮਾ ਹੈ ਪਰ ਅੱਜ ਵਸੋਂ ਵਿਚ ਵਾਧਾ ਅਤੇ ਜ਼ਰੂਰਤ ਤੋਂ ਵੱਧ ਵਸਤੁਆਂ ਦੇ ਉਪਯੋਗ ਦੇ ਕਾਰਨ ਭੂਮੀ ਦਾ ਵੱਧ ਸ਼ੋਸ਼ਣ ਕੀਤਾ ਜਾ ਰਿਹਾ ਹੈ ਅਤੇ ਭੂਮੀ ਤੇ ਦਬਾਅ ਵੱਧ ਰਿਹਾ ਹੈ ਅਤੇ ਸਾਡੇ ਸਾਧਨ ਖ਼ਤਰੇ ਵਿਚ ਹਨ । ਗ਼ਲਤ ਤਰੀਕੇ ਨਾਲ ਇਸਦੀ ਊਰਜਾ ਖ਼ਤਮ ਹੋ ਰਹੀ ਹੈ । ਮਿੱਟੀ ਦੇ ਸੂਖਮ ਜੀਵ ਖ਼ਤਮ ਹੋ ਰਹੇ ਹਨ । ਇਸ ਪ੍ਰਕਾਰ ਭੂਮੀ ਸਾਧਨਾਂ ਨੂੰ ਗੰਭੀਰ ਵਾਤਾਵਰਣ ਦੀਆਂ ਸਮੱਸਿਆਵਾਂ ਦਾ ਸਾਹਮਣਾ ਕਰਨਾ ਪੈਂਦਾ ਹੈ ।