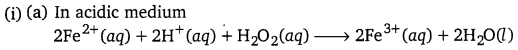Punjab State Board PSEB 11th Class Chemistry Important Questions Chapter 9 Hydrogen Important Questions and Answers.
PSEB 11th Class Chemistry Important Questions Chapter 9 Hydrogen Important Questions
Very Short Answer Type Questions
Question 1.
Name the isotope of hydrogen which contains equal number of protons and neutrons.
Answer:
Deuterium (\({ }_{1}^{2} \mathrm{H}\))
Number of protons (p) = number of electrons
= atomic number = 1
Number of neutrons (n) = mass number – atomic number
= 2 – 1 = 1 .
Question 2.
Why is the ionisation enthalpy of hydrogen higher than that of sodium?
Answer:
Both H and Na contain one electron in the valence shell. But the size of H is much smaller as compare to that of Na and hence, the ionisation enthalpy of hydrogen is much higher (1312 kJ mol-1) than that of Na (496 kJ mol-1).
![]()
Question 3.
What do you mean by 15 volume H2O2 solution?
Answer:
‘15 volume H2O2’ means 1 mL of a 15 volume H2O2 solution gives 15 mL of O2 at NTP.
Question 4.
Which isotope of hydrogen is radioactive?
Answer:
Tritium
Question 5.
Arrange H2, D2 and T2 in the decreasing order of their
(i) boiling points
(ii) heat of fusion
Answer:
(i) T2 > D2 > H2
(ii) T2 > D2 > H2
Question 6.
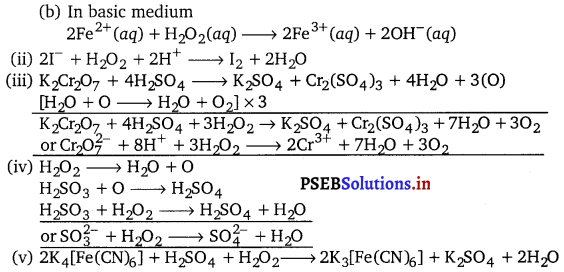
Write the Lewis structure of hydrogen peroxide.
Answer:
The Lewis structure of hydrogen peroxide is :
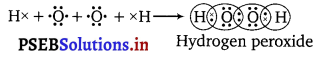
Question 7.
Write one chemical reaction for the preparation of D2O2.
Answer:
D2O2 is prepared by distillation of potassium persulphate (K2S2O8) with D2O.
![]()
Question 8.
Suggest a method to show the electronegative nature of hydrogen.
Answer:
When sodium hydride is electrolysed, hydrogen is evolved at anode, which shows its electronegative nature.
Question 9.
What type of bonds are broken when water evaporates.
Answer:
Intermolecular hydrogen bonds are broken when water evaporates.
Short Answer Type Questions
Question 1.
Describe the industrial applications of hydrogen dependent on
(i) the heat liberated when its atoms are made to combine on the surface of a metal.
(ii) its effect on the unsaturated organic systems in the presence of a catalyst.
(iii) its ability to combine with nitrogen under specific conditions.
Answer:
(i) Due to this property, hydrogen is used in atomic hydrogen welding/cutting torch.
(ii) Due to this property hydrogen is used for the manufacture of vanaspati ghee from edible oils such as cotton-seed oil, soyabean oil, corn oil etc.
![]()
(iii) Due to this property dihydrogen is used for the manufacture of ammonia (Haber’s process).
![]()
![]()
Question 2.
Why does water show high boiling point as compared to hydrogen sulphide? Give reasons for your answer.
Answer:
Water show high boiling point as compared to hydrogen sulphide due to high electronegativity of oxygen (EN = 3.5), water undergoes extensive H-bonding as a result of which water exists as associated molecule.
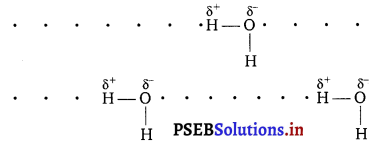
For breaking these hydrogen bond, a large amount of energy is needed and hence the boiling point of H2O is high. In other words, due to lower electronegativity of S (EN =2.5), hydrogen sulphide do not undergo H-bonding. Consequently, H2S exists as discrete molecule and hence its boiling point is much lower than that of H2O. That is why H2S is a gas at room temperature.
Question 3.
If a given sample of water has degree of hardness equal to 46 ppm. If entire hardness is due to MgSO4, how much MgSO4 is present per kg of water?
Answer:
Given, degree of hardness = 46 ppm
Which means that 106 g of sample require 46 g of CaCO3
∴ CaCO3 present in 1000 g of water = \(\frac{46 \times 1000}{10^{6}}\) = 46 x 10-3 g
1 mol (or 100 g) of CaC03 = 1 mol (or 120 g) of MgSO4
∴ 46 x 10-3 g of CaCO3 = \(\frac{120 \times 46 \times 10^{-3}}{100}\)g = 0.055 g or 55 mg
Question 4.
What are the advantages in using hydrogen as a fuel?
Answer:
Hydrogen as a fuel has the following advantages :
- It has high calorific value.
- During combustion, it does not produce smoke or any unpleasant fumes.
- It leaves no ash after burning. The only product of combustion is water.
- It does not pollute the air because no pollutant is produced during its combustion.
- It can be used in a fuel cell to generate electricity.
- It can be used in the internal combustion engines with slight modifications.
Question 5.
Calculate the volume strength of a 3% solution of H2O2
Answer:
100 mL of H2O2 solution contains H2O2 = 3 g
∴ 1000 mL of H2O2 solution will contains
H2O2 = \(\frac{3}{100}\) x 1000 = 30 g
Consider the chemical equation,
![]()
Now 68 g of H2O2 gives O2 at NTP = 22.7 L
∴ 30 g of H2O2 will give 02 at NTP = \(\frac{22.7}{68}\) x 30 = 10.014
But 30 g of H2O2 are present in 1000 mL of H2O2.
Hence, 1000 mL of H2O2 solution gives 02 at NTP = 1.0014 mL
∴ 1 mL of H2O2 solution will give O2 at NTP = \(\frac{10014}{1000}\)= 10.01 mL
Hence, the volume strength of 3% H202 solution = 10.01
![]()
Long Answer Type Questions
Question 1.
(i) (a) How would you prepare dihydrogen from water by using a reducing agent?
(b) How would you prepare dihydrogen from a substance other than water?
(c) How would you prepare very pure dihydrogen in the laboratory?
(ii) Write a short note on hydrogenation of vegetable oils.
Answer:
(i) (a) Sodium metal is a good reducing agent. It reduces water to hydrogen (or dihydrogen).
2H2O + 2Na → 2NaOH + H2(g)
(b) Dihydrogen can be obtained by treating zinc with dilute HCl
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
(c) Highly pure dihydrogen (hydrogen gas) can be prepared by the following methods :
I. Fairly pure hydrogen can be obtained by treating pure magnesium or pure aluminium with chemically pure H2SO4 or HCl diluted with distilled water. The gas is passed over P2O5 and is collected by the displacement of mercury.
Mg(s) + H2SO4(aq) > MgSO4(aq) + H2(g)
II. Highly pure hydrogen gas can be obtained by electrolysing a warm solution of Ba(OH)2 in a U-tube using nickel electrodes. The gas is purified by passing it over heated platinum gauze when traces of oxygen combine with hydrogen forming water. The gas is then dried by passing it over caustic potash sticks and phosphorus pentoxide. Hydrogen is finally adsorbed in palladium and the impurities remain unadsorbed. On heating palladium under reduced pressure pure hydrogen is liberated.
(ii) When oils like groundnut oil or cotton seed oil (which are unsaturated compound i.e., have double bond) are treated with hydrogen in the presence of nickel as catalyst, they get converted into edible fats like margarine and vanaspati ghee (which are saturated compounds). This reaction is called hydrogenation of vegetable oils or hardening of oils.
![]()
![]()
Question 2.
Give ion electron equations for the following reactions :
(i) Oxidation of ferrous ions to ferric ions by hydrogen peroxide both in acidic and basic media.
(ii) Oxidation of iodide ion to iodine by hydrogen peroxide in acidic medium.
(iii) Reduction of acidified potassium dichromate solution.
(iv) Oxidation of sulphurous acid to sulphuric acid.
(v) Oxidation of ferrocyanide ions to ferricyanide ions in acidic medium.
Answer: