Punjab State Board PSEB 12th Class Economics Book Solutions Chapter 27 भारत में शिक्षा, स्वास्थ्य तथा बेरोजगारी Textbook Exercise Questions, and Answers.
PSEB Solutions for Class 12 Economics Chapter 27 भारत में शिक्षा, स्वास्थ्य तथा बेरोजगारी
PSEB 12th Class Economics भारत में शिक्षा, स्वास्थ्य तथा बेरोजगारी Textbook Questions, and Answers
I. वस्तुनिष्ठ प्रश्न (Objective Type Questions)
प्रश्न 1.
भारत में साक्षरता की क्या स्थिति है ?
उत्तर-
भारत में 1951 में साक्षरता दर 18.3% थी। 2019 में साक्षरता दर बढ़कर 75% प्रतिशत हो गई है।
प्रश्न 2.
भारत में शिक्षा संस्थाओं की संख्या पर प्रकाश डालें।
उत्तर-
भारत में 2019-20 में 828 यूनिवर्सिटियां, 25938 प्रोफैशनल कॉलेज, 40,000 कॉलेज, 92275 सैकेण्डरी स्कूल, 139539 हाई स्कूल, 2 लाख 74 हज़ार से अधिक मिडिल स्कूल तथा 7 लाख 48 हज़ार से अधिक प्राइमरी स्कूल थे।
प्रश्न 3.
शिक्षा के व्यवसायीकरण से क्या अभिप्राय है ?
उत्तर-
शिक्षा के व्यवसायीकरण से अभिप्राय है शिक्षा के साथ किसी व्यवसाय की शिक्षा देना।
प्रश्न 4.
1986 की नई शिक्षा नीति के उद्देश्य बताओ।
उत्तर-
शत-प्रतिशत प्राइमरी शिक्षा प्रदान करना। ग्रामीण क्षेत्र में शिक्षा का प्रसार करना।
![]()
प्रश्न 5.
भारत में स्वतन्त्रता के पश्चात् जन्म दर तथा मृत्यु दर में क्या परिवर्तन हुआ है ?
उत्तर-
भारत में स्वतन्त्रता के पश्चात् जन्म दर तथा मृत्यु दर में बहुत कमी हुई है। 1951 में मृत्यु दर 18 प्रति हज़ार थी जोकि कम होकर 7.3 प्रति हज़ार रह गई है।
प्रश्न 6.
भारत में जीवन प्रत्याशा को स्पष्ट करो।
उत्तर-
भारत में स्वतन्त्रता के पश्चात् जीवन प्रत्याशा में काफ़ी वृद्धि हुई है। 1951 में भारत में लोगों की औसत आयु 41 वर्ष थी। 2019 में लोगों की औसत आयु बढ़कर 70.4 वर्ष हो गई है।
प्रश्न 7.
रोज़गार से क्या अभिप्राय है?
उत्तर-
वे सभी व्यक्ति (पुरुष तथा स्त्रियां) जो पारिश्रमिक के लिए किसी प्रकार की नौकरी अथवा अपना कार्य करते हैं, उनको रोज़गार पर लगे गिना जाता है।
प्रश्न 8.
श्रम शक्ति (Labour Force) से क्या अभिप्राय है ?
उत्तर-
जो व्यक्ति असल में काम करते हैं अथवा काम करने के इच्छुक हैं, उनको श्रम शक्ति कहा जाता है। श्रम शक्ति में 15 वर्ष से अधिक तथा 60 वर्ष से कम आयु के व्यक्तियों को गिना जाता है।
प्रश्न 9.
सामाजिक संरचना के तीन महत्त्वपूर्ण घटक बताएं।
उत्तर-
- शिक्षा
- स्वास्थ्य
- आवास।
प्रश्न 10.
लोगों के पढ़ने-लिखने तथा समझने की योग्यता को शिक्षा कहते हैं।
उत्तर-
सही।
प्रश्न 11.
भारत में शिक्षित बेरोज़गारी का मुख्य कारण शिक्षा को व्यवसाय से नहीं जोड़ा जाना है।
उत्तर-
सही।
प्रश्न 12.
2011 की जनगणना के अनुसार भारत में साक्षरता दर ………… प्रतिशत हो गई है।
(a) 64%
(b) 74.04%
(c) 84%
(d) इनमें से कोई नहीं।
उत्तर-
(b) 74.04%.
![]()
प्रश्न 13.
भारत में औसत जीवन अवधि …………….. वर्ष है।
(a) 53.5 वर्ष
(b) 63.5 वर्ष
(c) 73.5 वर्ष
(d) इनमें से कोई नहीं।
उत्तर-
(b) 63.5 वर्ष।
प्रश्न 14.
सम्भोग द्वारा जो बीमारियां एक व्यक्ति से दूसरे व्यक्ति में फैल जाती हैं उनको कहा जाता है।
उत्तर-
रजित रोग (Sexually Transmitted Diseases)
प्रश्न 15.
HIV से क्या अभिप्राय है ?
उत्तर-
ह्यूमन इमयुनो डैफीशेन्सी वायरस (HIV) का अर्थ शरीर में सफेद सैलज की कमी हो जाना जिनसे बीमारियों की रोकथाम होती है।
प्रश्न 16.
भारत में प्रारंभिक शिक्षा का कुल दाखिलों के अनुपात का अनुमान कैसे लगाया जाता है ?
उत्तर-
प्रारंभिक शिक्षा का कुल शिक्षा अनुपात-

प्रश्न 17.
सैकेण्डरी शिक्षा में शिक्षा के व्यवसायीकरण के लिए विशेष सफलता प्राप्त नहीं हुई है ?
उत्तर-
सही।
प्रश्न 18.
भारत में उच्च शिक्षा पर नियंत्रण तथा मार्गदर्शन कौन करता है ?
(a) केंद्र सरकार
(b) राज्य सरकार
(c) विश्वविद्यालय अनुदान आयोग
(d) उपरोक्त सभी।
उत्तर-
(c) विश्वविद्यालय अनुदान आयोग।
प्रश्न 19.
भारत में बेरोज़गारी का एक कारण शिक्षा का व्यवसायीकरण न होना है।
उत्तर-
सही।
प्रश्न 20.
भारत में शिक्षा पर सरकार द्वारा किया जाने वाला व्यय संतोषजनक है।
उत्तर-
ग़लत।
प्रश्न 21.
भारत में शिक्षा पर अधिक व्यय कौन करता है ?
(a) केंद्र सरकार
(b) राज्य सरकार
(c) निजी संस्था
(d) माता-पिता।
उत्तर-
(b) राज्य सरकार।
![]()
प्रश्न 22.
एड्ज़ (AIDS) से क्या अभिप्राय है ?
उत्तर-
एड्ज़ से अभिप्राय (एकुआयर्ड इमीनियो डैफीसेन्सी सिंड्रोम) रोगों से लड़ने की शक्ति की घाट।
प्रश्न 23.
औद्योगीकरण सेहत के लिए कैसे हानिकारक होता है ?
उत्तर-
औद्योगीकरण द्वारा वायु प्रदूषण, जल प्रदूषण और ध्वनि प्रदूषण फैलता है जो कि सेहत के लिए हानिकारक होता है।
प्रश्न 24.
भारत में बेरोज़गारी कितनी है ?
उत्तर-
भारत में 6.1% बेरोज़गार हैं।
प्रश्न 25.
भारत में शिक्षत बेरोज़गार कितने है ?
उत्तर-
भारत में 11.4% शिक्षत बेरोज़गार हैं।
II. अति लयु उत्तरीय प्रश्न (Very Short Answer Type Questions)
प्रश्न 1.
भारत में शिक्षा की स्थिति पर संक्षेप नोट लिखो।
उत्तर-
इस समय भारत में 74.04 प्रतिशत लोग साक्षर हैं। स्वतन्त्रता के पश्चात् शिक्षा के प्रसार की ओर अधिक ध्यान दिया गया है।
- साधारण शिक्षा का प्रसार-शिक्षित लोगों की संख्या में वृद्धि हुई है। भारत में 82.14% पुरुष तथा 65.46% स्त्रियां साक्षर हैं।
- शिक्षा संस्थाएं-भारत में स्कूल, कॉलेज तथा यूनिवर्सिटियों की संख्या में बहुत वृद्धि हुई है।
प्रश्न 2.
श्रम शक्ति तथा मानवीय शक्ति में अन्तर बताओ।
उत्तर-
- श्रम शक्ति उन व्यक्तियों की संख्या होती है जो वास्तव में काम पर लगे होते हैं अथवा काम करने के लिए तैयार होते हैं। कार्य शक्ति उन व्यक्तियों की संख्या होती है, जोकि वास्तव में कार्य पर लगे हुए होते हैं। उनमें उन व्यक्तियों को शामिल नहीं किया जाता, जो कार्य करने के लिए तैयार होते हैं।
- श्रम शक्ति में उन व्यक्तियों को शामिल करते हैं, जो देश में कार्य करने के लिए उपलब्ध होते हैं। कार्य शक्ति में उन व्यक्तियों को शामिल किया जाता है, जो वास्तव में काम पर लगे होते हैं।
प्रश्न 3.
रोज़गार से क्या अभिप्राय है ?
उत्तर-
रोज़गार का अर्थ है आर्थिक क्रिया में लगे होना। वह सभी व्यक्ति (पुरुष तथा स्त्रियां) जो किसी प्रकार के पारिश्रमिक के लिए किसी प्रकार की नौकरी अथवा अपना कार्य करते हैं को रोज़गार पर लगे हुए गिना जाता है। जब हम रोज़गार पर लगे व्यक्तियों की बात करते हैं तो उन व्यक्तियों को शामिल किया जाता है जो 15-60 वर्ष की आयु के हैं। 15 वर्ष से कम तथा 60 वर्ष से ऊपर की आयु के लोगों को कार्य शक्ति (Work Force) में शामिल नहीं किया जाता।
प्रश्न 4.
शहरी बेरोज़गारी पर टिप्पणी लिखें।
उत्तर-
शहरी बेरोज़गारी-भारत में 2017-18 में 991 लाख रजिस्टर्ड बेरोज़गार थे। इनमें औद्योगिक क्षेत्र के बेरोज़गार, शिक्षित बेरोज़गार जिनमें लड़के तथा लड़कियां शामिल हैं, उनमें बेरोज़गारी पाई जाती है। ग्रामीण तथा शहरी क्षेत्र में निम्नलिखित किस्म की बेरोज़गारी पाई जाती है –
- खुली बेरोज़गारी-जब काम करने योग्य श्रम शक्ति काम करना चाहती है परन्तु उनको काम नहीं मिलता तो उसको खुली बेरोज़गारी कहा जाता है।
- अल्प बेरोज़गारी-नैशनल सैम्पल सर्वे अनुसार यदि किसी व्यक्ति को साप्ताहिक 28 घण्टे काम प्राप्त होता है तो उसको अति अल्प बेरोजगार कहा जाता है। 29 से 42 घण्टे साप्ताहिक काम करने वाले श्रमिक को सीमित अल्प बेरोज़गार कहते हैं। जब कोई व्यक्ति किसी अन्य कार्य में अधिक उत्पादन कर सकता है तो वह वर्तमान कार्य में अल्प बेरोज़गार है।
- संरचनात्मक बेरोज़गारी-किसी देश में संरचनात्मक ढांचे में परिवर्तन हो जाए अर्थात् तकनीक तथा मांग में परिवर्तन के कारण बेरोज़गारी उत्पन्न हो जाए तो इसको संरचनात्मक बेरोज़गारी कहते हैं।
प्रश्न 5.
बेरोज़गारी के आर्थिक प्रभाव बताएँ।
उत्तर-
बेरोज़गारी के आर्थिक प्रभाव-
- जब बेरोज़गारी होती है तो मानवीय शक्ति का पूर्ण प्रयोग नहीं होता।
- देश में उत्पादन कम हो जाता है।
- देश में प्रति व्यक्ति पैदावार कम हो जाती है।
- पूंजी निर्माण के कम होने से निवेश पर बुरा प्रभाव पड़ता है।
![]()
III. लघु उत्तरीय प्रश्न (Short Answer Type Question)
प्रश्न 1.
भारत में शिक्षा की स्थिति पर संक्षेप नोट लिखो।
उत्तर-
सन् 2011 में भारत में 74.04 प्रतिशत लोग साक्षर थे। स्वतन्त्रता के पश्चात शिक्षा प्रणा की ओर अधिक ध्यान दिया गया है।
- साधारण शिक्षा का प्रसार-शिक्षित लोगों की संख्या 74.04% हो गई है। भारत में 82.14%, पुरुष तथा 65.46% स्त्रियां साक्षर हैं।
- शिक्षा संस्थाएं-भारत में स्कूल, कॉलेज तथा यूनिवर्सिटियों की संख्या में बहुत वृद्धि हुई है।
- विद्यार्थियों की संख्या-विद्यार्थियों की संख्या 2011 में बढ़कर 2 करोड़ हो गई है।
- शिक्षा का व्यवसायीकरण-शिक्षा को व्यवसाय आधारित बनाया जा रहा है।
प्रश्न 2.
भारत में स्वास्थ्य स्थिति का संक्षेप वर्णन करो।
उत्तर-
भारत की स्वास्थ्य स्थिति के सूचक इस प्रकार हैं –
- जन्म दर-भारत में 1951 में जन्म दर 40 प्रति हज़ार से घट कर 2019-20 में 17 प्रति हज़ार रह गई है।
- मृत्यु दर-मृत्यु दर 1951 में 18 प्रति हज़ार थी जोकि 2019-20 में घटकर 7.3 प्रति हज़ार रह गई है।
- जीवन स्तर-भारत में औसत आयु 1951 में 41 वर्ष प्रति हज़ार थी जोकि 2019-20 में 70.4 वर्ष हो गई है।
- अस्पताल तथा डॉक्टरों की संख्या-भारत में 70000 अस्पताल हैं, जिनमें कुल 8 लाख 890130 डॉक्टर तथा 19 लाख 4 हज़ार नर्से हैं।
प्रश्न 3.
श्रम पूर्ति तथा श्रम शक्ति में अन्तर बताओ।
उत्तर-
- श्रम पूर्ति का अर्थ मज़दूरी की दर के विभिन्न स्तर पर काम करने के लिए तैयार होते हैं। श्रम शक्ति का अर्थ वह मजदूरों की संख्या है जोकि काम कर रही है अथवा काम करने को तैयार है, परन्तु श्रम शक्ति का सम्बन्ध मज़दूरी की दर से नहीं होता।
- श्रम पूर्ति अधिक अथवा कम हो सकती है, जब मज़दूरी में परिवर्तन होता है, इसका माप मानवीय दिन (8 घण्टे) के अनुसार किया जाता है। श्रम शक्ति का अर्थ मज़दूरों की संख्या से होता है। इसको मानवीय दिन (8 घण्टे) द्वारा मापा नहीं जाता।
IV. दीर्घ उत्तरीय प्रश्न (Long Answer Type Questions)
प्रश्न 1.
स्वतन्त्रता के पश्चात् भारत में शिक्षा के विकास की व्याख्या करो। (Explain the status of Education in India after Independence.)
अथवा
भारत में वर्तमान शिक्षा स्थिति का वर्णन करो। शिक्षा सम्बन्धी सरकार की नीति को स्पष्ट करो।
(Explain the present education condition of India. Discuss the Policy of the Government regarding Educations.)
उत्तर-
स्वतन्त्रता के पश्चात् भारत में शिक्षा के विकास को बहुत महत्त्व दिया गया है। 1950-51 में शिक्षा पर 114 करोड़ रुपए व्यय किए गए थे। 2019-20 में शिक्षा के क्षेत्र के लिए 1.78 लाख करोड़ रुपए व्यय करने का लक्ष्य है। भारत में शिक्षा प्रणाली की वर्तमान स्थिति अग्रलिखित अनुसार है –
- साधारण शिक्षा का प्रसार-भारत में 1951 में कुल जनसंख्या का 16% भाग शिक्षित था। 2019-20 में शिक्षित लोगों की संख्या बढ़कर 74.4% हो गई है। इस प्रकार शिक्षा का प्रसार अधिक हुआ है। पुरुषों में शिक्षित लोग 82.14% तथा स्त्रियों में 65.46% प्रतिशत हैं।
- शिक्षा संस्थाएं तथा विश्वविद्यालय-भारत में इस समय (2019-20) 900 विश्वविद्यालय (Universities) तथा अनुसन्धान केन्द्र हैं। 25938 प्रोफेशनल शिक्षा संस्थाएं तथा 40000 कॉलेज हैं। सीनियर सैकेण्डरी स्कूलों की संख्या 233517, मिडिल स्कूल 18 लाख 10 हज़ार तथा प्राइमरी स्कूलों की संख्या 18 लाख 12 हज़ार से अधिक है। भारत में प्राइमरी शिक्षा निःशुल्क तथा अनिवार्य है।
- विद्यार्थियों की संख्या-1951 में 6 वर्ष से 11 वर्ष की आयु के 42 प्रतिशत विद्यार्थी स्कूल जाते थे। अब 80 प्रतिशत से अधिक विद्यार्थी स्कूल जाते हैं। विश्वविद्यालय में पढ़ने वाले विद्याथिर्यों की संख्या 1951 में 3.5 लाख थी जोकि 2019 में बढ़कर 2.9 करोड़ हो गई है।
- शिक्षा का व्यवसायीकरण-भारत में केन्द्र सरकार ने 1986 की नई शिक्षा नीति अनुसार सैकेण्डरी स्तर पर शिक्षा के व्यवसायीकरण की योजना आरम्भ की है। व्यावसायिक शिक्षा प्राप्त विद्यार्थियों के लिए निजी क्षेत्र, सार्वजनिक क्षेत्र तथा उनके स्व-रोज़गार के लिए योजनाबद्ध नीतियां बनाई गई हैं।
- प्रौढ़ तथा स्त्रियों की शिक्षा-भारत में बड़ी आयु के अनपढ़ पुरुषों तथा स्त्रियों के लिए शिक्षा को प्रौढ़ शिक्षा कहा जाता है। भारत में 15 वर्ष से 35 वर्ष के 11 करोड़ अनपढ़ हैं। सन् 2019-20 में 5.2 लाख लोगों के लिए शिक्षा का प्रबन्ध किया है। भारत में स्त्रियों की संख्या की ओर विशेष ध्यान दिया गया है।
- स्कूलों में विज्ञान का विषय-1988 के पश्चात् स्कूलों में विज्ञान की शिक्षा आरम्भ की गई है। इसका उद्देश्य शिक्षा के स्तर का सुधार करना तथा वैज्ञानिक दृष्टिकोण उत्पन्न करना है।
सरकार की शिक्षा नीति-भारत में नई शिक्षा नीति की घोषणा 1986 में की गई। इस नीति अनुसार केन्द्र तथा राज्य सरकारें शिक्षा पर व्यय सकल घरेलू उत्पाद का 6 प्रतिशत करेंगी। दसवीं योजना में शिक्षा पर 24908 करोड़ रु० व्यय किए गए। 2020-21 में शिक्षा पर 360000 करोड़ रु० व्यय होने का अनुमान है। नई नीति में निम्नलिखित बातों पर जोर दिया गया है-
- देश में सभी लोगों के लिए प्राथमिक शिक्षा प्रदान करना। इस उद्देश्य के लिए सर्वशिक्षा अभियान नवम्बर 2000 में आरम्भ किया गया। इस उद्देश्य के लिए 2020 में GDP का 3.9% भाग व्यय किया गया।
- जुलाई 2019 में लड़कियों की शिक्षा के लिए सर्व शिक्षा अभियान के स्थान पर “प्राथमिक स्तर पर लड़कियों की शिक्षा के लिए राष्ट्रीय प्रोग्राम” की घोषणा की है । इस योजना के अधीन पिछड़े क्षेत्रों में जहां स्त्री संख्या कम है, उन क्षेत्रों में स्त्री शिक्षा का विस्तार किया जाएगा।
- पिछड़ी जातियों तथा जनजाति की लड़कियों को प्राथमिक शिक्षा प्रदान करने के लिए ग्रामीण क्षेत्रों में रिहायशी स्कूल स्थापित करने का निर्णय लिया गया है। इस उद्देश्य के लिए 2014-15 में कस्तूरबा गांधी बालिका विद्यालय स्थापित करने के लिए बाहरवीं के वर्ष 2019-20 में 27 हज़ार करोड़ रु० व्यय करने का लक्ष्य है जिससे 750 रिहायशी स्कूल स्थापित किए जाएंगे।
- सरकार ने प्रत्यक्ष तथा अप्रत्यक्ष करों पर 3 प्रतिशत अतिरिक्त कर (Cess) लगाया है, जिसको प्राथमिक शिक्षा कोष का नाम दिया गया है। इस कर द्वारा 2019-20 में 2500 करोड़ रु० व्यय किए गए।
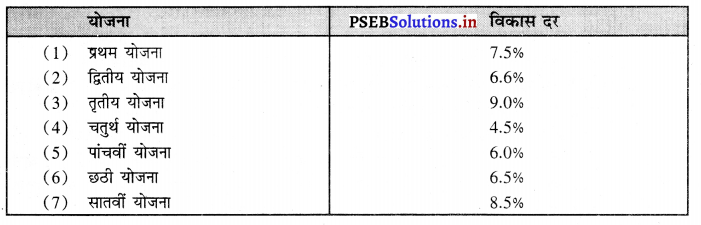
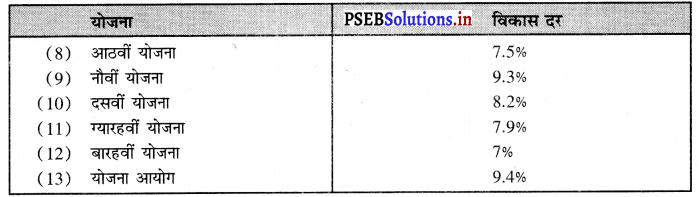
- तकनीकी शिक्षा के प्रसार पर भी जोर दिया गया है। बारहवीं योजना (2012-17) में स्कूल शिक्षा पर 360000 करोड़ अथवा उच्च शिक्षा पर 99300 लाख करोड़ रु० व्यय किये गए।
![]()
प्रश्न 2.
भारत में वर्तमान स्वास्थ्य स्थिति का वर्णन करो। इस सम्बन्धी सरकार की नीति स्पष्ट करो।
(Explain the present status of Health in India. Explain the Policy of Government in this regard.)
उत्तर-
किसी देश के साधनों में मानवीय साधनों को सबसे महत्त्वपूर्ण माना जाता है। किसी देश का आर्थिक विकास इस बात पर निर्भर करता है कि उस देश के मानवीय साधनों का शिक्षा, स्वास्थ्य, जीवन स्तर इत्यादि के रूप में कितना विकास हुआ है। प्रो० ड्रकर के अनुसार, “मनुष्यों के बिना पूंजी व्यर्थ होती है। पूंजी के बिना भी लोग पहाड़ों को हिलाने की शक्ति रखते हैं।” स्वतन्त्रता के पश्चात् भारत में मानवीय स्वास्थ्य पर बहुत बल दिया गया है। इसका अनुमान निम्नलिखित सूची पत्र द्वारा लगाया जा सकता है
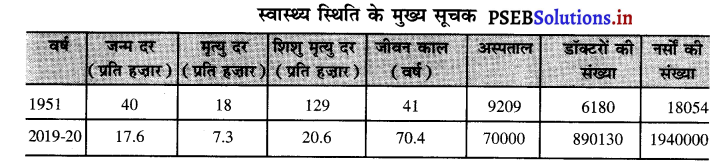
भारत में स्वास्थ्य स्थिति का अनुमान ऊपर दी सूची से लगाया जा सकता है।
- जन्म दर-भारत में 1951 में जन्म दर 40 प्रति हज़ार थी जोकि 2019-20 में घटकर 17.6 प्रति हज़ार रह गई है। जन्म दर में कमी आई है जोकि जनसंख्या की वृद्धि पर रोक लगाने के लिए अच्छी है।
- मृत्यु दर-भारत में मृत्यु दर भी काफ़ी घट गई है। 1951 में मृत्यु दर 18 प्रति हज़ार थी। यह घटकर 2019-20 में 7.3 प्रति हज़ार रह गई है। इसका कारण स्वास्थ्य सुविधाओं का विकास है।
- शिशु मृत्यु दर-भारत में बच्चों के जन्म समय शिशुओं की मृत्यु हो जाती थी। 1951 में शिशु मृत्यु दर 129 प्रति हजार से घटकर 2019-20 में 26.6 प्रति हज़ार रह गई है। यह भी स्वास्थ्य सुविधाओं में वृद्धि का प्रतीक है।
- जीवन प्रत्याशा-भारत में औसत जीवन प्रत्याशा में वृद्धि हुई है। 1951 में औसत जीवन प्रत्याशा 41 वर्ष थी। 2019-20 में औसत जीवन प्रत्याशा 70.4 वर्ष हो गई है। पुरुषों की जीवन प्रत्याशा 67 वर्ष तथा स्त्रियों की जीवन प्रत्याशा 74.4 वर्ष है।
- अस्पतालों की संख्या-भारत में 1951 में अस्पतालों की संख्या 70000 थी। यह संख्या 2019-20 में बढ़कर 593320 हो गई है। इससे लोगों को विभिन्न बीमारियों का इलाज करवाना आसान हो गया है।
- डॉक्टरों तथा नौं की संख्या- भारत में डॉक्टरों की संख्या में भी निरन्तर वृद्धि हुई है। 1951 में 61810 डॉक्टर थे। 2019-20 में डॉक्टरों की संख्या बढ़कर 890130 हो गई है। देश के प्रत्येक प्रान्त में मेडिकल कलेज स्थापित हो गए हैं। मरीजों की देखभाल तथा इलाज में नौं का योगदान भी महत्त्वपूर्ण होता है। 1951 में भारत में नों की संख्या 18054 थी जोकि बढ़कर 2019-20 में 1940000 हो गई।
स्वास्थ्य सम्बन्धी समस्याएं- भारत में स्वास्थ्य सम्बन्धी सुविधाओं की कुछ समस्याएं है जैसे कि –
- गांवों तथा शहरों में स्वास्थ्य सुविधाएं एक समान नहीं है। गांवों में स्वास्थ्य सुविधाओं की कमी है।
- एड्ज, शूगर, ब्ल्ड प्रैशर बीमारियों में वृद्धि हो रही है।
- निजीकरण के कारण स्वास्थ्य सम्भाल की लागत बढ़ रही है।
- साफ-सुथरे वातावरण तथा स्वास्थ्य सुविधाओं की कमी पाई जाती है।
स्वास्थ्य सम्बन्धी नीति-दसवीं पंचवर्षीय योजना में स्वास्थ्य सुविधाओं पर सकल घरेलू उत्पाद का 2-3 प्रतिशत हिस्सा व्यय करने की सिफ़ारिश की गई है। इस नीति के मुख्य उद्देश्य निम्नलिखित हैं
- 2019-20 में एड्ज़ पर नियन्त्रण करने के लिए 2100 करोड़ रुपए व्यय किए गए।
- प्राइमरी स्वास्थ्य केन्द्र गांवों में स्थापित किए गए हैं, जिनकी संख्या 2019-20 में 192166 हो गई है।
- 2019-20 के बजट में स्वास्थ्य सुविधाओं पर 2998 करोड़ रु० व्यय किए गए।
- देश के 19 राज्यों में कुष्ठ की बीमारी को समाप्त किया गया है। बारहवीं योजना के अन्त तक इस बीमारी को समाप्त कर दिया जाएगा।
- देश में 2019-20 में 14 से 16 मिलियन पुरुष, स्त्रियां तथा बच्चे एड्ज़/एच० आई० वी० के शिकार हो चुके थे। यह देश में स्वास्थ्य सुविधाओं की कमी का सूचक है।
- भारत में आयुर्वेद, योगा, यूनानी तथा होमियोपैथी को विकसित किया जा रहा है। इन इलाजों के विकास के लिए बारहवीं योजना में 13999 करोड़ रु० व्यय किए जाएंगे।
- बारहवीं योजना में स्वास्थ्य पर 842481 करोड़ रु० व्यय किये जाएंगे।
प्रश्न 3.
भारत में बेरोज़गारी की किस्में बताओ। बेरोज़गारी के आर्थिक तथा सामाजिक प्रभाव बताओ।
(Explain the types of unemployment in India. Discuss the Economic and Social Effects and Education.)
उत्तर-
भारत में बेरोजगारी दिन-प्रतिदिन बढ़ रही है। जब एक मनुष्य काम पर नहीं होता परन्तु वह मनुष्य वर्तमान मज़दूरी की दर पर काम करना चाहता है तो उनको बेरोज़गार कहा जाता है। भारत में 2000 में 26.6 मिलियन, 2004 में 34.3 मिलियन तथा 2009 में 28 मिलियन तथा 2019-20 में 20.9 मिलियन श्रम शक्ति बेरोज़गार थी। भारत में बेरोज़गारी का वर्गीकरण निम्नलिखित अनुसार किया जा सकता है –
1. ग्रामीण बेरोज़गारी-गांवों में दो-तिहाई श्रम शक्ति स्व:रोज़गार पर कृषि उत्पादन में लगी हुई है। भारत में 2016 में 8.52% लोग बेरोज़गार थे। परन्तु गांवों में छिपी बेरोज़गारी भी पाई जाती है। छिपी बेरोज़गारी का अर्थ है कि कृषि उत्पादन में एक खेत पर पांच व्यक्तियों की आवश्यकता है परन्तु आठ व्यक्ति काम पर लगे हुए हैं, यदि 3 व्यक्तियों को अन्य काम पर लगाया जाए परन्तु कृषि के उत्पादन में कोई कमी न आए तो तीन श्रमिकों को छिपे बेरोज़गार कहा जाता है। इसी तरह गांवों में कृषि उत्पादन मौसमी कार्य है। इस कारण मौसमी बेरोज़गारी भी पाई जाती है।
2. शहरी बेरोज़गारी- भारत में 2019-20 में 11.4% बेरोज़गार थे। इनमें औद्योगिक क्षेत्र के बेरोज़गार, शिक्षित बेरोज़गार जिनमें लड़के तथा लड़कियां शामिल हैं, उनमें बेरोज़गारी पाई जाती है। ग्रामीण तथा शहरी क्षेत्र में निम्नलिखित किस्म की बेरोज़गारी पाई जाती है –
- खुली बेरोज़गारी-जब काम करने योग्य श्रम शक्ति काम करना चाहती है परन्तु उनको काम नहीं मिलता तो उसको खुली बेरोज़गारी कहा जाता है।
- अल्प बेरोज़गारी-नैशनल सैम्पल सर्वे अनुसार यदि किसी व्यक्ति को साप्ताहिक 28 घण्टे काम प्राप्त होता है तो उसको आत अल्प बेरोज़गार कहा जाता है। 29 से 42 घण्टे साप्ताहिक काम करने वाले श्रमिक को सीमित अल्प गेज़गार कहते हैं। जब कोई व्यक्ति किसी अन्य कार्य में अधिक उत्पादन कर सकता है तो वह नमान कार्य में अल्प बेरोज़गार है।
- संरचनात्मक बेरोज़गारी-किसी देश में संरचनात्मक ढांचे में परिवर्तन हो जाए अर्थात् तकनीक तथा मांग में परिवर्तन के कारण बेरोज़गारी उत्पन्न हो जाए तो इसको संरचनात्मक बेरोज़गारी कहते हैं।
- अस्थिर बेरोज़गारी-अस्थिर बेरोज़गारी देश में श्रम की गतिहीनता तथा श्रम बाज़ार की अपूर्णता के कारण उत्पन्न होती है, जब मजदूरों को बाज़ार का ज्ञान नहीं होता तो इस प्रकार की बेरोज़गारी उत्पन्न हो जाती है।
- चक्रीय बेरोज़गारी-किसी देश में मन्दीकाल के कारण उत्पन्न हुई बेरोज़गारी को चक्रीय बेरोज़गारी कहा जाता है।
- मौसमी बेरोज़गारी-मौसम में परिवर्तन होने से कुछ उद्योग बन्द हो जाते हैं तो इस प्रकार की बेरोज़गारी को मौसमी बेरोज़गारी कहा जाता है।
![]()
बेरोजगारी के आर्थिक प्रभाव-
- जब बेरोज़गारी होती है तो मानवीय शक्ति का पूर्ण प्रयोग नहीं होता।
- देश में उत्पादन कम हो जाता है।
- देश में प्रति व्यक्ति पैदावार कम हो जाती है।
- पूंजी निर्माण के कम होने से निवेश पर बुरा प्रभाव पड़ता है।
बेरोज़गारी पर सामाजिक प्रभाव-
- जीवन की गुणवत्ता कम हो जाती है।
- समाज में अमीर तथा गरीब वर्ग उत्पन्न हो जाते हैं।
- आर्थिक तथा सामाजिक असमानता में वृद्धि होती है।
- सामाजिक अशान्ति फैल जाती है जैसे कि नक्सलवादी तथा आतंकवादी समाज में अशान्ति उत्पन्न करते हैं।
प्रश्न 4.
भारत में शहरी तथा ग्रामीण रोजगार के लिए सरकार द्वारा कौन-सी योजनाएं बनाई गई हैं ? इनका उल्लेख करें।
(Explain the programs of the Government of India regarding rural and urban employment.)
उत्तर-
भारत में रोजगार की वृद्धि के लिए सरकार द्वारा निम्नलिखित पग उठाए गए हैंशहरी रोज़गार में वृद्धि के लिए उठाए गए पग-
- संगठित क्षेत्रों का विकास-भारत में निजी क्षेत्र तथा सार्वजनिक क्षेत्र में अर्थात् उद्योग, खनिज, यातायात निर्माण इत्यादि में बहुत-से लोगों को रोजगार प्रदान करवाया जा रहा है।
- रोज़गार के दफ़्तर-सरकार ने रोज़गार की सुविधाएं बढ़ाने के लिए 915 रोज़गार दफ़्तर स्थापित किए हैं। सरकार बेरोज़गारी भत्ता देने के बारे भी विचार कर रही है।
- शिक्षित लोगों को स्व:रोज़गार-भारत में शिक्षित लोगों को स्व:रोज़गार दिलवाने के लिए सरकार बहुत-सी योजनाएं लागू कर रही है। शिक्षित लोगों को ऋण की सुविधाएं प्रदान की जा रही हैं।
- रोज़गार गारण्टी योजना-कुछ राज्यों जैसे कि महाराष्ट्र, पश्चिमी बंगाल, केरल तथा राजस्थान इत्यादि में रोज़गार गारण्टी योजना आरम्भ की है। महाराष्ट्र में 2019 में 326 करोड़ रु० व्यय किए गए।
ग्रामीण रोज़गार में वृद्धि के लिए उठाए गए पग-
1. रोजगार के लिए धमाकेदार प्रोग्राम-1971 में बेरोज़गारी तथा अल्प बेरोज़गारी को दूर करने के लिए प्रत्येक जिले में श्रम सघन योजना आरम्भ की गई। इससे प्रत्येक परिवार के कम-से-कम एक व्यक्ति को रोज़गार प्रदान किया जाता है। इस योजना पर प्रत्येक वर्ष 100 करोड़ रु० व्यय किए गए हैं।
2. राष्ट्रीय ग्रामीण रोजगार प्रोग्राम-1980-81 में पहले काम के बदले अनाज योजना बनाई गई थी जिसका नाम बदलकर राष्ट्रीय ग्रामीण रोजगार प्रोग्राम रखा गया। इस योजना के तीन उद्देश्य थे
- गांवों में अधिक रोज़गार के अवसर पैदा करना।
- ग्रामीण लोगों का जीवन स्तर ऊँचा उठाना
- लोगों की आय में वृद्धि करना।
3. स्व:रोज़गार के लिए प्रशिक्षण-ग्रामीण स्वरोजगार प्रशिक्षण योजना (TRYSEM) 1979 में आरम्भ की गई। इस योजना के अन्तर्गत 12 लाख युवकों को प्रशिक्षण दिया गया। प्रत्येक युवक को 3000 अथवा 5000 रु० तक की आर्थिक सहायता भी दी गई ताकि वह अपना कार्य आरम्भ कर सकें।
4. जवाहर रोज़गार योजना-28 अप्रैल, 1989 में स्वर्गीय प्रधानमन्त्री श्री राजीव गांधी ने जवाहर रोज़गार योजना (JRY) आरम्भ की। इस योजना अधीन प्रत्येक गरीब ग्रामीण परिवार के एक सदस्य को 50 से 100 दिनों तक का रोज़गार देना है। इस योजना में स्त्रियों के लिए 30 प्रतिशत हिस्सा सुरिक्षत किया गया। इस योजना पर 2019-20 में 7200 करोड़ रु० व्यय किए गए।
5. जवाहर ग्राम समृद्धि योजना-यह योजना अप्रैल 1999 में आरम्भ की गई। इसमें केन्द्र तथा राज्य सरकारों का योगदान 75:25 की अनुपात में रखा गया। इस योजना का उद्देश्य गांवों में पक्की नालियां, गलियां, धर्मशालाएं इत्यादि चिरकालीन चलने वाले भण्डारों का विकास करना है। 2018-19 में केन्द्र सरकार ने 61500 करोड़ की राशि का योगदान इस योजना में पाया।
6. स्वर्ण जयन्ती ग्राम स्व:रोज़गार-यह योजना एकीकृत ग्रामीण विकास प्रोग्राम तथा स्वरोजगार के लिए प्रशिक्षण योजना को शामिल करके तैयार की गई। इसमें पहले चलाई जा रही योजनाओं को चालू रखना इसका उद्देश्य रखा गया।
7. सम्पूर्ण ग्रामीण रोजगार योजना-यह योजना 1 सितम्बर, 2001 में आरम्भ की गई। जवाहर ग्राम समृद्धि योजना तथा रोज़गार आश्वासन योजना को मिला दिया गया। इस योजना का उद्देश्य मज़दूरों को रोजगार के अधिक अवसर प्रदान करना, क्षेत्रीय आर्थिक तथा सामाजिक दशाओं का विकास करना है। इस योजना अधीन 2018-19 में 82 लाख टन चावल तथा 44 लाख टन गेहूं का वितरण किया गया।
8. प्रधानमन्त्री रोज़गार योजना-यह योजना शिक्षित बेरोजगार युवकों के लिए बनाई गई है। 2017-18 में इस योजना द्वारा 88 लाख युवकों को रोजगार दिया जा चुका है।
9. रोज़गार गारण्टी एक्ट 2005-यह योजना सितम्बर 2004 में लागू की गई है। इस योजना अनुसार प्रत्येक ग्रामीण, शहरी तथा निर्धन परिवार में से एक सदस्य को 100 दिनों का काम प्रदान किया जाएगा। 2018-19 में इस योजना पर व्यय करने के लिए 30 हज़ार करोड़ रुपये रखे गए हैं जोकि सकल घरेलू उत्पाद का 1.3% हिस्सा है।
10. प्रधानमन्त्री कौशल विकास योजना-NDA सरकार ने 2015 में कौशल विकास योजना का निर्माण किया। इसमे बेरोजगार युवकों को कौशल प्रदान करके रोजगार दिया जाएगा। इसमें अब तक 4.30 लाख बेरोजगारों को शिक्षा प्रदान की जा चुकी है।
11. दीन दयाल उपाध्याय ग्राम कौशल योजना-यह योजना 2019-20 में आरम्भ की गई इस योजना द्वारा 2.78 लाख ग्रामीण बेरोजगार युवकों को सिखलाई दी गई है।
12. बारहवीं योजना और रोज़गार-भारत की बारहवीं योजना (2012-17) के अन्त तक श्रम शक्ति में वृद्धि 65 करोड़ होगी तथा 42 करोड़ श्रम शक्ति के लिये रोज़गार प्रदान किया जाएगा। योजना के अन्त तक 5% बेरोज़गारी रह जाने की सम्भावना है। इस योजना में बेरोजगारों को शिल्प (Skill) सिखलाई देकर देश का विकास किया जाएगा।
![]()
13. बजट 2020-21-बजट 2020-21 में वित्त मंत्री निर्मला सीतारमण ने ग्रामीण क्षेत्र में रोजगार की वृद्धि के लिए 69000 करोड़ की राशि व्यय करने का लक्ष्य रखा है।