Punjab State Board PSEB 12th Class Chemistry Book Solutions Chapter 14 Biomolecules Textbook Exercise Questions and Answers.
PSEB Solutions for Class 12 Chemistry Chapter 14 Biomolecules
PSEB 12th Class Chemistry Guide Biomolecules InText Questions and Answers
Question 1.
What are monosaccharides?
Answer:
Monosaccharides are carbohydrates which cannot be further hydrolysed to simpler molecules. The general formula of monosaccharides is (CH2O)n where n = 3-7.
Question 2.
What are reducing sugars?
Answer:
Carbohydrates which reduce Fehling’s solution to red precipitate of Cu2O or Tollen’s reagent to metallic Ag are called reducing sugars. All monosaccharides (both aldoses and ketoses) and disaccharides except sucrose are reducing sugars.
Question 3.
Write two main functions of carbohydrates in plants.
Answer:
Two main functions of carbohydrates in plants are:
- Polysaccharides such as starch serve as storage molecules.
- Cellulose, a polysaccharide, is used to build the cell wall.
Question 4.
Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose
Answer:
Monosaccharides: Ribose, 2-deoxyribose, galactose, fructose
Disaccharides: Maltose, lactose.
![]()
Question 5.
What do you understand by the term glycosidic linkage?
Answer:
Glycosidic linkage refers to the linkage formed between two monosaccharide units through an oxygen atom by the loss of a water molecule. For example, in a sucrose molecule, two monosaccharide units, α-D-glucose and β-D-fructose, are joined together by a glycosidic linkage.
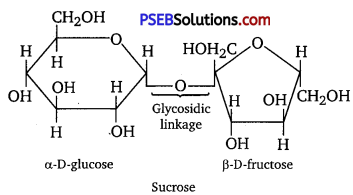
Question 6.
What is glycogen? How is it different from starch?
Answer:
Glycogen is a carbohydrate (polysaccharide). In animals, carbohydrates are stored as glycogen. Starch is a carbohydrate consisting of two components-amylose (15 – 20%) and amylopectin (80 – 85%). However, glycogen consists of only one component whose structure is similar to amylopectin. Also, glycogen is more branched than amylopectin. Glycogen chain consists of 10-14 glucose units whereas amylopectin chains consist of 20-25 glucose units.
Question 7.
What are the hydrolysis products of (i) sucrose and (ii) lactose?
Answer:
(i) On hydrolysis, sucrose gives one molecule of α-D glucose and one molecule of β-D-fructose.
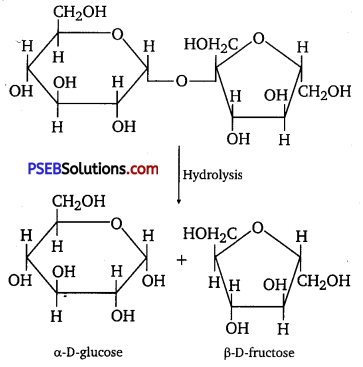
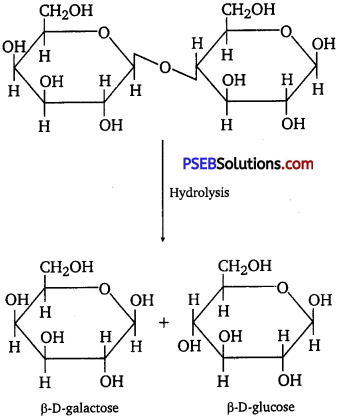
(ii) On hydrolysis, lactose gives β-D-galactose and β-D-glucose.
Question 8.
What is the basic structural difference between starch and cellulose?
Answer:
Starch consists of two components-amylose and amylopectin. Amylose is a long linear chain of ≅α-D-(+)-glucose units joined by C1 -C4 glycosidic linkage (α-link).
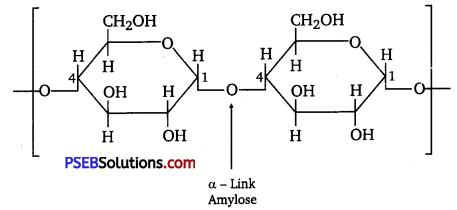
Amylopectin is a branched-chain polymer of []α-D-glucose units, in which the chain is formed by C1 -C4 glycosidic linkage and the branching occurs by C1 – C6 glycosidic linkage.
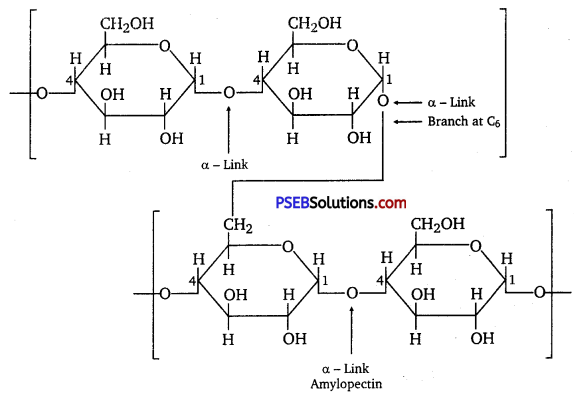
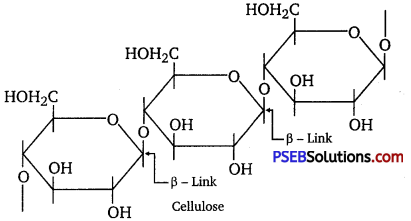
On the other hand, cellulose is a straight-chain polysaccharide of β-D-glucose units joined by C1– C4 glycosidic linkage (β-link).
![]()
Question 9.
What happens when D-glucose is treated with the following reagents?
(i) HI
(ii) Bromine water
(iii) HNO3
Answer:
(i) HI s On prolonged heating with HI, glucose forms n-hexane.

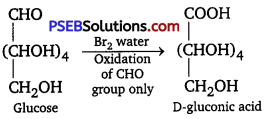
(ii) Br2 water: Glucose gets oxidised (gluconic acid) on reaction with a mild oxidising agent like bromine water.
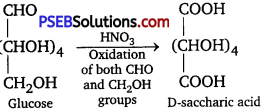
(iii) HNO3: On oxidation with nitric acid, glucose yields a dicarboxylic acid, that is saccharic acid.
Question 10.
Enumerate the reactions of D-glucose which cannot be explained by its open-chain structure.
Answer:
- Aldehydes give 2, 4-DNP test, Schiff’s test, and react with NaHSO4 to form the hydrogen sulphite addition product. However, glucose does not undergo these reactions.
- The pentaacetate of glucose does not react with hydroxylamine. This indicates that a free -CHO group is absent from glucose.
- Glucose exists in two crystalline forms-α and β. The α-form (m.p. = 419 K) crystallises from a concentrated solution of glucose at 303 K and the β-form (m.p = 423 K) crystallises from a hot and saturated aqueous solution at 371 K. This behaviour cannot be explained by the open chain structure of glucose.
Question 11.
What are essential and non-essential amino acids? Give two examples of each type.
Answer:
Essential amino acids are required by the human body, but they cannot be synthesised in the body. They must be taken through food, e.g., valine and leucine. Non-essential amino acids are also required by the human body, but they can be synthesised in the body e.g., glycine and alanine.
Question 12.
Define the following as related to proteins
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation.
Answer:
(i) Peptide linkage: The amide formed between —COOH group of one molecule of an amino acid and -NH2 group of another molecule of the amino acid by the elimination of a water molecule is called a peptide linkage.
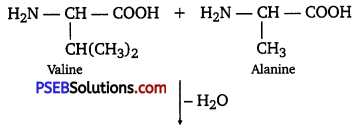
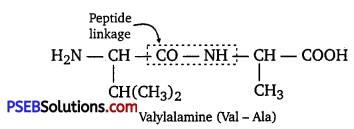
(ii) Primary structure: The primary structure of protein refers to the specific sequence in which various amino acids are present in it, i.e., the sequence of linkages between amino acids in a polypeptide chain. The sequence in which amino acids are arranged is different in each protein. A change in the sequence creates a different protein.
(iii) Denaturation: In a biological system, a protein is found to have a unique 3-dimensional structure and a unique biological activity. In such a situation, the protein is called native protein. However, when the native protein is subjected to physical changes such as change in temperature or chemical changes such as change in pH, its H-bonds are disturbed.
This disturbance unfolds the globules and uncoils the helix. As a result, the protein loses its biological activity. This loss of biological activity by the protein is called denaturation. During denaturation, the secondary and the tertiary structures of the protein get destroyed, but the primary structure remains unaltered. One of the examples of denaturation of proteins is the coagulation of egg white when an egg is boiled.
![]()
Question 13.
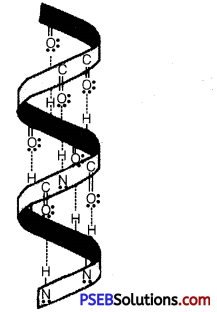
What are the common types of secondary structures of proteins?
Answer:
There are two common types of secondary structure of proteins:
(i) α- helix structure: In this structure, the -NH group of an amino acid residue forms H-bond with the img group of the adjacent turn of the right-handed screw (α-helix).
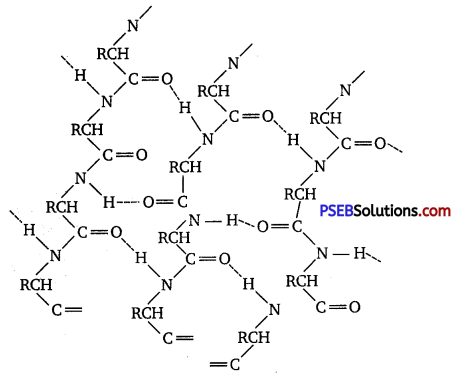
(ii) β-pleated sheet structure: This structure is called so because it looks like the pleated folds of drapery. In this structure, all the peptide chains are stretched out to nearly the maximum extension and then laid side by side. These peptide chains are held together by intermolecular hydrogen bonds.
Question 14.
What type of bonding helps in stabilising the α-helix structure of proteins?
Answer:
The α-helix structure of proteins is stabilised by intramolecular H-bonding between C-O of one amino acid residue and the N-H of the fourth amino acid residue in the chain.
Question 15.
Differentiate between globular and fibrous proteins.
Answer:
| Globular proteins | Fibrous proteins |
| 1. These are water-soluble proteins. | These are water-insoluble proteins. |
| 2. These are spherical in shape. | These are linear in shape. |
| 3. Globular proteins are highly unstable. | Fibrous proteins are stable to moderate changes in temperature and pH. |
Question 16.
How do you explain the amphoteric behaviour of amino acids?
Answer:
In aqueous solution, the carboxyl group of an amino acid can lose a proton and the amino group can accept a proton to give a dipolar ion known as zwitterion.
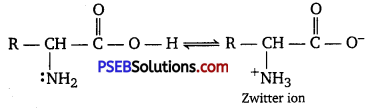
Therefore, in zwitterionic form, the amino acid can act both as an acid and as a base.
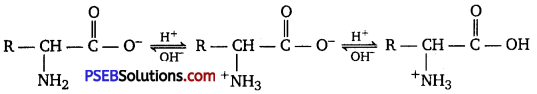
Thus, amino acids show amphoteric behaviour.
Question 17.
What are enzymes?
Answer:
Enzymes are proteins that catalyse biological reactions. They are very specific in nature and catalyse only a particular reaction for a particular substrate. Enzymes are usually named after the particular substrate or class of substrate and sometimes after the particular reaction. For example, the enzyme used to catalyse the hydrolysis of maltose into glucose is named as maltase.
![]()
Again, the enzymes used to catalyse the oxidation of one substrate with the simultaneous reduction of another substrate are named as oxidoreductase enzymes. The name of an enzyme ends with ‘ – ase’.
![]()
Question 18.
What is the effect of denaturation on the structure of proteins?
Answer:
During denaturation, 2° and 3° structures of proteins are destroyed but 1° structure remains intact. Due to denaturation, the globular proteins (soluble in H20) are converted into fibrous proteins (insoluble in H2O) and their biological activity is lost. For example, boiled egg which contains coagulated proteins cannot be hatched.
Question 19.
How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Answer:
Vitamins are classified into two groups depending upon their solubility in water or fat.
- Water-soluble vitamins: These include vitamin B-complex (B1, B2, B5, i.e., nicotinic acid, B6, B12, pantothenic acid, biotin, i.e., vitamin H and folic acid) and vitamin C.
- Fat-soluble vitamins: These include vitamins A, D, E and K. These are stored in liver and adipose tissues (fat-storing tissues). Vitamin K is responsible for coagulation of blood.
Question 20.
Why are vitamin A and vitamin C essential to us? Give their important sources.
Answer:
Vitamin A: Vitamin A is essential for us because its deficiency can cause xerophthalmia (hardening of cornea of eye) and night blindness. Sources: Carrots, fish liver oil, butter and milk.
Vitamin C: Vitamin C is essential for us because its deficiency causes scurvy (bleeding gums) and pyorrhea (loosening and bleeding of teeth).
Sources: Amla, citrus fruits and green leafy vegetables.
Question 21.
What are nucleic acids? Mention their two important functions.
Answer:
Nucleic acids are biomolecules which are found in the nuclei of all living cells in the form of nucleoproteins or chromosomes (proteins containing nuclei acids as the prosthetic group).
These are of two types: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).
The two main functions of nucleic acids are :
- DNA is responsible for transmission of hereditary effects from one generation to another. This is because of the unique property of replication during cell division and the transfer of two identical DNA strands to the daughter cells.
- DNA and RNA are responsible for synthesis of all proteins essential for the growth and maintenance of our body. Actually, the proteins are synthesised by various RNA molecules (rRNA, mRNA and tRNA) in the cell but the message for the synthesis of a particular protein is present in DNA.
Question 22.
What is the difference between a nucleoside and a nucleotide?
Answer:
A nucleoside is formed when 1-position of pyrimidine (cytosine, thymine or uracil) or 9-position of purine (guanine or adenine) base is connected to C-1 of sugar (ribose or deoxyribose) by a β-linkage. Hence, in general, nucleosides may be represented as: Sugar-Base.
A nucleotide contains all the three basic compounds of nucleic acids, i.e., a phosphoric acid group, a pentose sugar and a nitrogenous base. These are obtained by esterification of C’5– OH group of the pentose sugar by phosphoric acid.
Question 23.
The two strands in DNA are not identical but are complementary. Explain.
Answer:
In the helical structure of DNA, the two strands are held together by hydrogen bonds between specific pairs of bases. Cytosine forms hydrogen bond with guanine, while adenine forms hydrogen bond with thymine. As a result, the two strands are complementary to each other.
Question 24.
Write the important structural and functional differences between DNA and RNA.
Answer:
Structural differences
| DNA | RNA |
| (i) The sugar present in DNA is 2-deoxy-D-(-)-ribose. | The sugar present in RNA is D-(-)-ribose. |
| (ii) DNA contains cytosine and thymine as pyrimidine bases. | RNA contains cytosine and uracil as pyrimidine bases. |
| (iii) DNA has a double-stranded a-helix structure. | RNA has a single-stranded a-helix structure. |
| (iv) DNA molecules are very large; their molecular mass may vary from 6 x 106 -16 x 106 u. | RNA molecules are much smaller with molecular mass ranging from 20,000 to 40,000 u. |
Functional differences:
| (i) DNA has unique property of replication. | RNA usually does not replicate. |
| (ii) DNA controls the transmission of hereditary effects. | RNA controls the synthesis of proteins. |
Question 25.
What are the different types of RNA found in the cell?
Answer:
Three types of RNA present in the cell are :
- Messenger RNA (m-RNA)
- Ribosomal RNA (r-RNA)
- Transfer RNA (t-RNA)
![]()
Chemistry Guide for Class 12 PSEB Biomolecules Textbook Questions and Answers
Question 1.
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six-membered ring compounds) are insoluble in water. Explain.
Answer:
Glucose and sucrose have hydroxyl (-OH) groups (5 in glucose and 8 in sucrose) which form strong hydrogen bonds with water molecules and hence these are soluble in water. Cyclohexane and benzene are non-polar compounds and do not have hydroxyl groups and hence they are not able to form hydrogen bonding with water molecules and, therefore, these are not soluble in water.
Question 2.
What are the expected products of hydrolysis of lactose?
Answer:
Lactose is composed of β-D galactose and β-D glucose. Thus, on hydrolysis, it gives D-(+)- galactose and D-(+)- glucose.
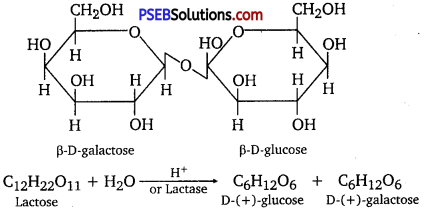
Question 3.
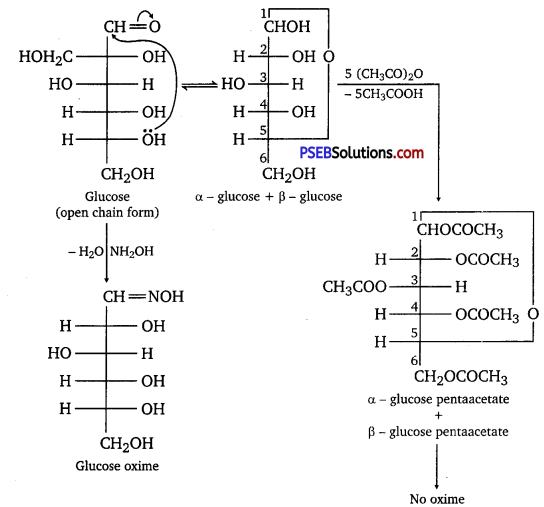
How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
Answer:
D-glucose reacts with hydroxylamine (NH2OH) to form an oxime because of the presence of aldehydic (-CHO) group or carbonyl carbon. This happens as the cyclic structure of glucose forms an open-chain structure in an aqueous medium, which then reacts with NH2OH to give an oxime.
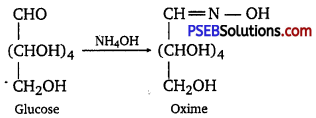
But pentaacetate of D-glucose does not react with NH2OH. This is because pentaacetate does not form an open-chain structure.
![]()
Question 4.
The melting points and solubility in water of amino acids are generally higher than that of the corresponding halo acids. Explain.
Answer:
Both acidic (carboxyl) as well as basic (amino) groups are present in the same molecule of amino acids. In aqueous solutions, the carboxyl group can lose a proton and the amino group can accept a proton, thus giving rise to a dipolar ion known as a zwitterion.
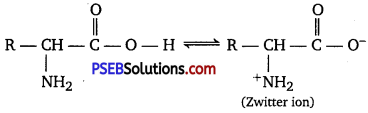
Due to this dipolar behaviour, they have strong electrostatic interactions within them and with water. But halo-acids do not exhibit such dipolar behaviour. For this reason, the melting points and the solubility of amino acids in water is higher than those of the corresponding halo-acids.
Question 5.
Where does the water present in the egg go after boiling the egg?
Answer:
When an egg is boiled, the proteins present inside the egg get denatured and coagulated. After boiling the egg, the water present in it is absorbed by the coagulated protein through H-bonding.
Question 6.
Why cannot vitamin C be stored in our body?
Answer:
Because vitamin C are water-soluble vitamins and so these are readily excreted through urine and hence cannot be stored in our body.
Question 7.
What products would be formed when a nucleotide from DNA containing thymine is hydrolysed?
Answer:
When a nucleotide from the DNA containing thymine is hydrolysed, thymine β-D-2-deoxyribose and phosphoric acid are obtained as products.
Question 8.
When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA?
Answer:
On complete hydrolysis of RNA six compounds are isolated. These compounds are adenine, guanine, cytosine, uracil, D-ribose and phosphoric acid. There is, no relationship among the quantities of different bases obtained on hydrolysis of RNA suggests that RNA is single-stranded. If it would have been double-stranded like DNA the complementary pairing of bases would have given equal proportion of complementary bases.