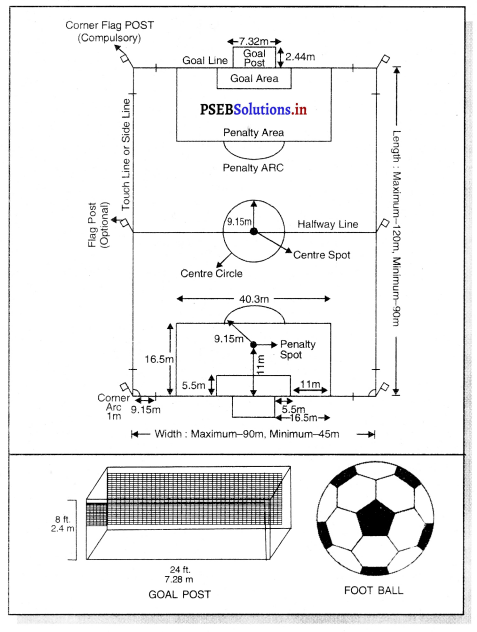
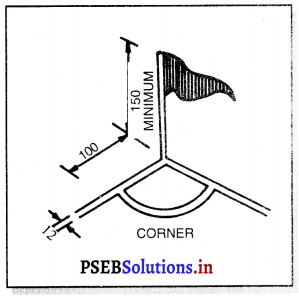
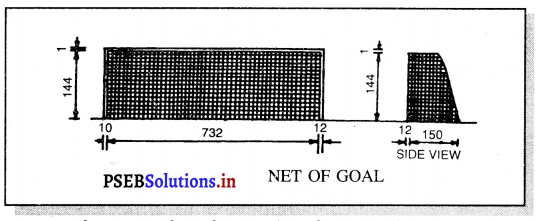
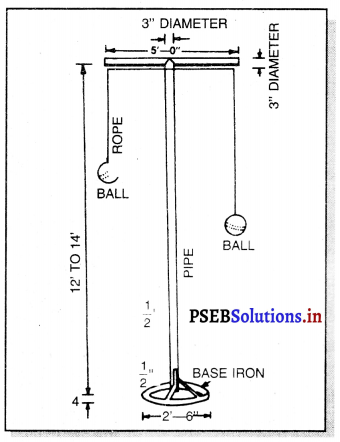
Punjab State Board PSEB 11th Class Physical Education Book Solutions Chapter 2 ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਅਤੇ ਇਸ ਦੀ ਮਹੱਤਤਾ Textbook Exercise Questions, and Answers.
PSEB Solutions for Class 11 Physical Education Chapter 2 ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਅਤੇ ਇਸ ਦੀ ਮਹੱਤਤਾ
Physical Education Guide for Class 11 PSEB ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਅਤੇ ਇਸ ਦੀ ਮਹੱਤਤਾ Textbook Questions and Answers
ਅਭਿਆਸ ਦੇ ਪ੍ਰਸ਼ਨਾਂ ਦੇ ਉੱਤਰ
ਪ੍ਰਸ਼ਨ 1.
ਜ਼ਿੰਦਗੀ ਦੁਆਰਾ ਪੈਦਾ ਹੋਈਆਂ ਚੁਣੌਤੀਆਂ ਨੂੰ ਕਿਸ ਤਰ੍ਹਾਂ ਨਜਿੱਠਿਆ ਜਾ ਸਕਦਾ ਹੈ ? (How the Challenges faced in our life is resolved.)
ਉੱਤਰ-
ਆਧੁਨਿਕ ਯੁਗ ਪਦਾਰਥਵਾਦੀ ਯੁੱਗ ਹੈ । ਅੱਜ ਹਰ ਇਨਸਾਨ ਪਦਾਰਥ ਇਕੱਠੇ ਕਰਨ ਦੀ ਦੌੜ ਵਿਚ ਏਨਾ ਉਲਝਿਆ ਹੋਇਆ ਹੈ ਕਿ ਉਸ ਕੋਲ ਆਪਣੇ ਲਈ ਵੀ ਸਮਾਂ ਨਹੀਂ ਹੈ । ਇਹ ਯੁੱਗ ਇਨਸਾਨ ਲਈ ਤਨਾਵ, ਦਬਾਵ ਤੇ ਚਿੰਤਾ ਦਾ ਯੁੱਗ ਬਣ ਕੇ ਰਹਿ ਗਿਆ ਹੈ । ਇਸ ਲਈ ਜ਼ਿਆਦਾਤਰ ਵਿਅਕਤੀ ਖੁਸ਼ੀ ਨਾਲ ਭਰਪੂਰ ਅਤੇ ਫਲਦਾਇਕ ਜੀਵਨ ਨਹੀਂ ਗੁਜ਼ਾਰ ਰਹੇ ਹਨ | ਅਜਿਹੇ ਰੁਝੇਵਿਆਂ ਭਰੇ ਜੀਵਨ ਕਾਰਨ ਹਰੇਕ ਵਿਸ਼ੇ ਦੀਆਂ ਧਾਰਨਾਵਾਂ ਵਿੱਚ ਤਬਦੀਲੀਆਂ ਹੋ ਰਹੀਆਂ ਹਨ ਜਿਸ ਨਾਲ ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਦੇ ਖੇਤਰ ਵਿੱਚ ਵੀ ਵਿਸਥਾਰ ਹੋਇਆ ਹੈ । ਅੱਜ ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਦਾ ਸੰਬੰਧ ਸਰੀਰਿਕ ਕਸਰਤਾਂ ਦੇ ਨਾਲ-ਨਾਲ ਮਨੁੱਖ ਦੇ ਜੀਵਨ ਦੇ ਹਰ ਪੱਖ ਨਾਲ ਹੈ | ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਇਨਸਾਨ ਨੂੰ ਆਪਣਾ ਰੁਝੇਵਿਆਂ ਭਰਿਆ ਜੀਵਨ ਠੀਕ ਢੰਗ ਨਾਲ ਬਤੀਤ ਕਰਨ ਲਈ ਉਸ ਦੀ ਮੱਦਦ ਕਰਦੀ ਹੈ ।
ਇਸ ਨਾਲ ਇਨਸਾਨ ਸਰੀਰਿਕ ਕੌਸ਼ਲਾਂ, ਸਰੀਰ ਦੀ ਜਾਣਕਾਰੀ, ਜੀਵਨ-ਮੁੱਲ ਅਤੇ ਸਿਹਤਮੰਦ ਜੀਵਨ ਬਤੀਤ ਕਰਨ ਦੇ ਗੁਣ ਪ੍ਰਾਪਤ ਕਰਦਾ ਹੈ । ਇਨ੍ਹਾਂ ਗੁਣਾਂ ਨਾਲ ਵਿਅਕਤੀ ਵਿੱਚ ਹੌਸਲਾ ਪੈਦਾ ਹੁੰਦਾ ਹੈ ਅਤੇ ਉਹ ਜੀਵਨ ਦੀਆਂ ਔਕੜਾਂ ਨੂੰ ਹੌਸਲੇ ਨਾਲ ਨਜਿੱਠਣ ਦੇ ਕਾਬਿਲ ਬਣਦਾ ਹੈ । ਇਸ ਤਰ੍ਹਾਂ ਅੱਜ ਦੇ ਮਸ਼ੀਨੀ ਯੁੱਗ ਅਤੇ ਕਿਰਿਆਹੀ ਜ਼ਿੰਦਗੀ ਦੁਆਰਾ ਪੈਦਾ ਹੋਈਆਂ ਚੁਣੌਤੀਆਂ ਨੂੰ ਕੇਵਲ ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਰਾਹੀਂ ਅਤੇ ਸਰੀਰਿਕ ਕਸਰਤਾਂ ਕਰਨ ਨਾਲ ਹੀ ਨਜਿੱਠਿਆ ਜਾ ਸਕਦਾ ਹੈ ।
![]()
ਪ੍ਰਸ਼ਨ 2.
ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਦੀ ਪਰਿਭਾਸ਼ਾ ਲਿਖੋ । (Define Physical Education.)
ਉੱਤਰ-
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੀ ਕੋਈ ਇੱਕ ਪਰਿਭਾਸ਼ਾ ਦੇਣਾ ਬਹੁਤ ਮੁਸ਼ਕਲ ਕੰਮ ਹੈ । ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਦੇ ਰੂਪਾਂ, ਕੰਮਾਂ ਅਤੇ ਉਸ ਸੰਬੰਧੀ ਵਿਚਾਰਧਾਰਾਵਾਂ ਵਿੱਚ ਲਗਾਤਾਰ ਤਬਦੀਲੀਆਂ ਰਹੀਆਂ ਹਨ | ਪੁਰਾਣੇ ਸਮੇਂ ਤੋਂ ਲੈ ਕੇ ਅੱਜ ਤਕ ਇਸ ਨੇ ਵਿਅਕਤੀ, ਸਮਾਜ ਅਤੇ ਦੇਸ਼ ਦੀਆਂ ਲੋੜਾਂ ਅਨੁਸਾਰ ਇਕ ਵਿਸ਼ੇਸ਼ ਸਰੀਰਕ ਸਿੱਖਿਆ ਪ੍ਰਣਾਲੀ ਨੂੰ ਅਪਣਾਇਆ ਹੈ। ਅਤੇ ਇਸ ਯੁੱਗ ਵਿਚ ਪ੍ਰਣਾਲੀ ਬਦਲ ਗਈ ਹੈ । ਇਹੀ ਕਾਰਨ ਹੈ ਕਿ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੀਆਂ ਵੱਖ-ਵੱਖ ਪਰਿਭਾਸ਼ਾਵਾਂ ਦਿੱਤੀਆਂ ਗਈਆਂ ਹਨ ।
ਚਾਰਲਸ ਏ. ਬੂਚਰ ਦੇ ਵਿਚਾਰ ਅਨੁਸਾਰ, “ਸਰੀਰਕ ਸਿੱਖਿਆ ਸਮੁੱਚੀ ਸਿੱਖਿਆ ਦੀ ਕਿਰਿਆ ਦਾ ਇਕ ਅਨਿੱਖੜਵਾਂ ਅੰਗ ਹੈ ਅਤੇ ਉੱਦਮ ਦੇ ਖੇਤਰ ਵਿਚ ਇਸ ਦਾ ਉਦੇਸ਼ ਸਰੀਰਕ, ਮਾਨਸਿਕ, ਭਾਵਾਤਮਕ ਅਤੇ ਸਮਾਜਿਕ ਰੂਪ ਤੋਂ ਸੰਪੂਰਨ ਨਾਗਰਿਕਾਂ ਦਾ, ਅਜਿਹੀਆਂ ਸਰੀਰਕ ਕਿਰਿਆਵਾਂ ਦੇ ਮਾਧਿਅਮਾਂ ਰਾਹੀਂ ਵਿਕਾਸ ਕਰਨਾ ਜਿਨ੍ਹਾਂ ਦੀ ਚੋਣ ਇਨ੍ਹਾਂ ਉਦੇਸ਼ਾਂ ਨੂੰ ਸਾਹਮਣੇ ਰੱਖ ਕੇ ਕੀਤੀ ਜਾਏ ।” (Charles A. Bucher)
(“Physical Education, an integral part of the total education process, is a field of endeavour which has as its aim the development of physically, mentally, emotionally and socially fit citizens through the medium of the physical activities, which have been selected with a view of realise the outcomes.”) ।
ਸਾਧਾਰਨ ਸਿੱਖਿਆ ਅਤੇ ਸਰੀਰਕ ਸਿੱਖਿਆ ਬਾਰੇ ਉਪਰੋਕਤ ਵਿਚਾਰਾਂ ਰਾਹੀਂ ਇਹ ਗੱਲ ਸਪੱਸ਼ਟ ਹੋ ਜਾਂਦੀ ਹੈ ਕਿ ਸਰੀਰਕ ਸਿੱਖਿਆ ਅਤੇ ਸਾਧਾਰਨ ਸਿੱਖਿਆ ਦੋਹਾਂ ਦੇ ਉਦੇਸ਼ ਇਕ ਦੂਜੇ ਤੋਂ ਵੱਖਰੇ ਨਹੀਂ ਹਨ । ਸਾਧਾਰਨ ਸਿੱਖਿਆ ਰਾਹੀਂ ਇਨ੍ਹਾਂ ਉਦੇਸ਼ਾਂ ਨੂੰ ਜਮਾਤ ਦੇ ਕਮਰੇ ਵਿਚ ਦਿੱਤੇ ਗਏ ਭਾਸ਼ਣਾਂ ਰਾਹੀਂ ਅਤੇ ਅਧਿਐਨ ਰਾਹੀਂ ਪ੍ਰਾਪਤ ਕੀਤਾ ਜਾਂਦਾ ਹੈ ਜਦ ਕਿ ਸਰੀਰਕ ਸਿੱਖਿਆ ਵਿਚ ਇਨ੍ਹਾਂ ਉਦੇਸ਼ਾਂ ਨੂੰ ਸਰੀਰਕ ਕਿਰਿਆਵਾਂ ਰਾਹੀਂ ਪ੍ਰਾਪਤ ਕੀਤਾ ਜਾਂਦਾ ਹੈ ।
ਸਧਾਰਨ ਸਿੱਖਿਆ ਇਕ ਅਜਿਹਾ ਗਿਆਨ ਹੈ ਜੋ ਸਰੀਰ ਨਾਲ ਸੰਬੰਧ ਰੱਖਦਾ ਹੈ । ਸਰੀਰ ਦੀ ਬਣਤਰ, ਵਿਕਾਰ ਅਤੇ ਵਾਧੇ ਨੂੰ ਸੋਧ ਦਿੰਦਾ ਹੈ ਅਤੇ ਇਸ ਦਾ ਸਾਧਨ ਸਰੀਰਕ ਕਿਰਿਆਵਾਂ ਹੀ ਹਨ। ਇਸ ਬਾਰੇ ਵੱਖ-ਵੱਖ ਵਿਦਵਾਨਾਂ ਨੇ ਵੱਖਵੱਖ ਪਰਿਭਾਸ਼ਾਵਾਂ ਦਿੱਤੀਆਂ ਜਿਨ੍ਹਾਂ ਵਿਚੋਂ ਕੁਝ ਇਸ ਤਰ੍ਹਾਂ ਹਨ-
ਡੀ. ਓਬਰਟਿਊਫਰ ਦੇ ਅਨੁਸਾਰ, “ਸਰੀਰਕ ਸਿੱਖਿਆ ਉਨ੍ਹਾਂ ਸਾਰਿਆਂ ਤਜਰਬਿਆਂ ਦਾ ਜੋੜ ਹੈ ਜੋ ਕਿਸੇ ਵਿਅਕਤੀ ਨੂੰ ਸਰੀਰਕ ਹਰਕਤ ਰਾਹੀਂ ਪ੍ਰਾਪਤ ਹੁੰਦੇ ਹਨ ।
(“Physical education is the sum of those experiences which come to the individual through movement.”) (D. Oberteuffer)
ਆਰ. ਕੈਸਿਡੀ ਦੇ ਸ਼ਬਦਾਂ ਵਿਚ, “ਸਰੀਰਕ ਸਿੱਖਿਆ ਉਨ੍ਹਾਂ ਸਾਰੀਆਂ ਤਬਦੀਲੀਆਂ ਦਾ ਜੋੜ ਹੈ ਜੋ ਵਿਅਕਤੀ ਵਿਚ ਹਰਕਤ ਰਾਹੀਂ ਆਉਂਦੀਆਂ ਹਨ ।
(“’Physical education is the sum of change in the individual caused by experiences which bring in motor activity.”) (R. Cassidy)
ਜੇ. ਬੀ. ਨੈਸ਼ ਲਿਖਦੇ ਹਨ, “ਸਰੀਰਕ ਸਿੱਖਿਆ ਵਿੱਦਿਆ ਦੇ ਸਮੁੱਚੇ ਖੇਤਰ ਦਾ ਉਹ ਹਿੱਸਾ ਹੈ ਜੋ ਵੱਡੇ ਪੱਠਿਆਂ ਦੇ ਕਾਰਜਾਂ ਦੇ ਪ੍ਰਤੀਕਿਰਿਆਵਾਂ ਨਾਲ ਸੰਬੰਧ ਰੱਖਦਾ ਹੈ ।”
(“Physical education is that phase of the whole field of education that deals with big muscles activities and their related responses.”’) (J.B. Nash)
ਜੇ. ਐੱਫ. ਵਿਲੀਅਮਜ਼ ਦੇ ਅਨੁਸਾਰ, “ਸਰੀਰਕ ਸਿੱਖਿਆ ਵਿਅਕਤੀ ਦੀਆਂ ਕੁੱਲ ਸਰੀਰਕ ਕਿਰਿਆਵਾਂ ਦਾ ਜੋੜ ਹੈ ਜਿਹੜੀਆਂ ਕਿ ਆਪਣੀ ਭਿੰਨਤਾ ਅਨੁਸਾਰ ਚੁਣੀਆਂ ਜਾਂਦੀਆਂ ਹਨ ਅਤੇ ਆਪਣੇ ਮੰਤਵ ਅਨੁਸਾਰ ਵਰਤੀਆਂ ਜਾਂਦੀਆਂ ਹਨ ।’’
(“Physical education is the sum of man’s physical activities selected as to kind and conducted as to outcomes.”) (J.F. Williams)
ਚਾਰਲਸ ਏ. ਬੂਚਰ ਅਨੁਸਾਰ, “ਸਰੀਰਕ ਸਿੱਖਿਆ ਸਮੁੱਚੀ ਸਿੱਖਿਆ ਦੀ ਕਿਰਿਆ ਦਾ ਇਕ ਅਨਿੱਖੜਵਾਂ ਅੰਗ ਹੈ ਜਿਸ ਦਾ ਮੰਤਵ ਸਰੀਰਕ, ਮਾਨਸਿਕ, ਜਜਬਾਤੀ ਅਤੇ ਸਮਾਜੀ ਤੌਰ ਤੇ ਠੀਕ ਸ਼ਹਿਰੀ ਪੈਦਾ ਕਰਨਾ ਹੈ । ਇਸ ਟੀਚੇ ਤੇ ਪਹੁੰਚਣ ਲਈ ਸਰੀਰਕ ਕਿਰਿਆਵਾਂ ਦਾ ਸਥਾਨ ਚੁਣਿਆ ਗਿਆ ਹੈ ਤਾਂ ਜੋ ਇਸ ਟੀਚੇ ਨੂੰ ਪਾ ਸਕੀਏ ।”
(“Physical Education, an integral part of the total education process is a field of endeavour which has as its aim the development of physically, mentally, emotionally and socially fit citizens through the medium of the social activities, which have been selected with a view to realising these outcomes.”) (Charles A. Bucher)
ਜੇ. ਆਰ. ਸ਼ਰਮਨ ਅਨੁਸਾਰ ਸਰੀਰਕ ਸਿੱਖਿਆ ਵਿੱਦਿਆ ਦਾ ਉਹ ਅੰਗ ਹੈ ਜੋ ਸਰੀਰਕ ਕਿਰਿਆਵਾਂ ਰਾਹੀਂ ਵਿਅਕਤੀ ਦੇ ਆਚਰਨ ਤੇ ਤੌਰ-ਤਰੀਕਿਆਂ ਨੂੰ ਸੋਧ ਦਿੰਦਾ ਹੈ।”
(“Physical education is that part of education which takes place through activities which involve that motor mechanism of the human body and which results in the individual’s formulating behaviour patterns.”’) (J.R. Sharman)
ਐੱਚ. ਸੀ. ਚੱਕ ਦੇ ਅਨੁਸਾਰ, “ਸਰੀਰਕ ਸਿੱਖਿਆ, ਸਿੱਖਿਆ ਦੇ ਸਧਾਰਣ ਕਾਰਜਕ੍ਰਮ ਦਾ ਇਕ ਭਾਗ ਹੈ, ਜਿਸਦਾ ਸੰਬੰਧ ਸਰੀਰਕ ਕਿਆ ਕਲਾਪਾਂ ਦੁਆਰਾ ਬੱਚਿਆਂ ਦਾ ਵਾਧਾ, ਵਿਕਾਸ ਅਤੇ ਸਿੱਖਿਆ ਨਾਲ ਹੈ । ਇਹ ਸਰੀਰਕ ਕ੍ਰਿਆ ਕਲਾਪਾਂ ਦੁਆਰਾ ਬੱਚਿਆਂ ਦੀ ਸੰਪੂਰਨ ਸਿੱਖਿਆ ਹੈ । ਸਰੀਰਕ ਕਿਰਿਆਵਾਂ ਸਾਧਨ ਹਨ ਅਤੇ ਉਸਦੀ ਚੋਣ ਅਤੇ ਪ੍ਰਯੋਗ ਇਸ ਪ੍ਰਕਾਰ ਕੀਤੀ ਜਾਂਦੀ ਹੈ ਕਿ ਉਸਦਾ ਪ੍ਰਭਾਵ ਬੱਚੇ ਦੇ ਜੀਵਨ ਦੇ ਹਰੇਕ ਪਹਿਲ, ਸਰੀਰਕ ਮਾਨਸਿਕ, ਸੰਵੇਗਾਤਮਕ ਅਤੇ ਨੈਤਿਕ ਪੱਖ ‘ਤੇ ਪਵੇ ।”
(“Physical education is the part of general education programme, which is considered with growth, development and education of children through the medium of big muscle activities. It is the education of whole child by means of Physical activities. Physical activities are the tools. They are so selected and conducted as to influence every child’s life. Physically, mentally. emotionally and morally.”) (H.C. Buck) ।
ਸੀ. ਸੀ. ਕੋਵੇਲ ਦੇ ਅਨੁਸਾਰ, “ਸਰੀਰਕ ਸਿੱਖਿਆ ਵਿਅਕਤੀ ਖ਼ਾਸ ਦੇ ਸਮਾਜਿਕ ਵਿਵਹਾਰ ਵਿੱਚ ਉਹ ਪਰਿਵਰਤਨ ਹੈ। ਜੋ ਮਾਸਪੇਸ਼ੀਆਂ ਅਤੇ ਉਨ੍ਹਾਂ ਨਾਲ ਸੰਬੰਧਿਤ ਵੱਡੀ ਗਤੀਵਿਧੀ ਦੀ ਪ੍ਰੇਰਣਾ ਨਾਲ ਉਪਜਦਾ ਹੈ !”
(“Physical education is the social process of change in the behaviour of human organism, originating primarily from the stimulums of social, big muscle, play and related activities.”) (C.C. Cowell ) ।
ਸੀ. ਐੱਲ. ਬਰਾਉਨੇਲ ਦੇ ਅਨੁਸਾਰ, ਸਰੀਰਕ ਸਿੱਖਿਆ ਉਹ ਪਰੀਪੂਰਣ ਅਤੇ ਸੰਤੁਲਿਤ ਅਨੁਭਵਾਂ ਦਾ ਜੋੜ ਹੈ ਜੋ ਵਿਅਕਤੀ ਨੂੰ ਬਹੁਪੇਸ਼ੀ ਪ੍ਰਕਿਰਿਆਵਾਂ ਵਿੱਚ ਭਾਗ ਲੈਣ ਤੋਂ ਪ੍ਰਾਪਤ ਹੁੰਦੇ ਹਨ ਅਤੇ ਉਸਦੀ ਅਭਿਵਰਤੀ ਅਤੇ ਵਿਕਾਸ ਨੂੰ ਚਰਮਸੀਮਾ ਤੱਕ ਵਧਾਉਂਦੇ ਹਨ’’ ।
(“Physical education is the accumulation of wholesome experience through participation in large muscle activities that promote optimum growth and development.’)) (C.L. Brownell,) ।
ਭਾਰਤੀ ਸਰੀਰਕ ਸਿੱਖਿਆ ਅਤੇ ਮਨੋਰੰਜਨ ਦੇ ਕੇਂਦਰੀ ਸਲਾਹਕਾਰ ਬੋਰਡ ਦੀ ਪਰਿਭਾਸ਼ਾ ਅਨੁਸਾਰ, “ਸਰੀਰਕ ਸਿੱਖਿਆ, ਸਿੱਖਿਆ ਹੀ ਹੈ । ਇਹ ਉਹ ਸਿੱਖਿਆ ਹੈ ਜਿਹੜੀ ਬੱਚੇ ਦੇ ਸੰਪੂਰਨ ਵਿਅਕਤੀਤਵ ਲਈ ਜਾਂ ਉਸ ਦੀਆਂ ਸਰੀਰਕ ਕਿਰਿਆਵਾਂ ਰਾਹੀਂ ਸਰੀਰ, ਮਨ ਅਤੇ ਆਤਮਾ ਦੇ ਪੂਰਨ ਵਿਕਾਸ ਲਈ ਦਿੱਤੀ ਜਾਂਦੀ ਹੈ ।”
(“’Physical education is education. It is education through physical activities for the development of the total personality of the child to its perfection in body, mind and spirit.”) (Central Advisory Board of Physical Education and Recreation.)
ਉੱਪਰਲੀਆਂ ਸਾਰੀਆਂ ਪਰਿਭਾਸ਼ਾਵਾਂ ਦਾ ਅਧਿਐਨ ਕਰਨ ਮਗਰੋਂ ਹੇਠ ਲਿਖੀਆਂ ਗੱਲਾਂ ਸਾਹਮਣੇ ਆਉਂਦੀਆਂ ਹਨ-
- ਸਰੀਰਕ ਸਿੱਖਿਆ, ਸਿੱਖਿਆ ਦਾ ਹੀ ਇਕ ਅਨਿੱਖੜਵਾਂ ਅੰਗ ਹੈ ।
- ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਮਾਧਿਅਮ ਸਰੀਰਕ ਕਿਰਿਆਵਾਂ ਹੀ ਹਨ ।
- ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਮਨੋਰਥ ਕੇਵਲ ਸਰੀਰ ਨੂੰ ਸਿਹਤਮੰਦ ਅਤੇ ਸੁੰਦਰ ਬਣਾਉਣ ਦਾ ਹੀ ਨਹੀਂ ਹੈ । ਇਹ ਇਕ | ਅਜਿਹਾ ਸਿੱਖਿਆ ਪ੍ਰਬੰਧ ਹੈ ਜਿਸ ਨਾਲ ਮਨੁੱਖ ਦਾ ਬਹੁਮੁਖੀ ਵਿਕਾਸ ਹੋ ਸਕੇ ।
- ਅੱਜ ਦੀ ਸਰੀਰਕ ਸਿੱਖਿਆ ਯੋਜਨਾਬੱਧ ਹੈ ਅਤੇ ਵਿਗਿਆਨਿਕ ਸਿਧਾਂਤਾਂ ਉੱਤੇ ਆਧਾਰਿਤ ਹੈ ।
![]()
ਪਸ਼ਨ 3.
ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਦਾ ਕੀ ਟੀਚਾ ਹੈ ? (What is the aim of Physical Education ?)
ਉੱਤਰ-
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਟੀਚਾ (Aim of Physical Education)
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਟੀਚੇ ਸੰਬੰਧੀ ਵੱਖ-ਵੱਖ ਵਿਦਵਾਨਾਂ ਨੇ ਆਪਣੇ-ਆਪਣੇ ਢੰਗ ਨਾਲ ਆਪਣੇ ਵਿਚਾਰ ਪ੍ਰਗਟਾਏ ਹਨ । ਇਹਨਾਂ ਵਿਚੋਂ ਪ੍ਰਮੁੱਖ ਵਿਦਵਾਨਾਂ ਦੇ ਵਿਚਾਰ ਹੇਠਾਂ ਲਿਖੇ ਹਨ-
ਜੇ. ਐਫ. ਵਿਲੀਅਮਜ਼ ਦੇ ਅਨੁਸਾਰ, “ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਟੀਚਾ ਇਕ ਕੁਸ਼ਲ ਅਗਵਾਈ, ਉੱਚਿਤ ਸਹੂਲਤਾਂ ਅਤੇ ਕਾਫੀ ਸਮਾਂ ਦੁਆਉਣਾ ਹੈ ਜਿਸ ਨਾਲ ਵਿਅਕਤੀ ਜਾਂ ਸੰਗਠਨਾਂ ਨੂੰ ਇਸ ਤਰ੍ਹਾਂ ਦੀਆਂ ਸਥਿਤੀਆਂ ਵਿਚ ਭਾਗ ਲੈਣ ਦਾ ਅਵਸਰ ਮਿਲ ਸਕੇ ਜੋ ਸਰੀਰਕ ਰੂਪ ਨਾਲ ਆਨੰਦਦਾਇਕ, ਮਾਨਸਿਕ ਰੂਪ ਨਾਲ ਪ੍ਰੇਰਕ ਤੇ ਸੰਤੋਖਜਨਕ ਅਤੇ ਸਮਾਜਿਕ ਰੂਪ ਨਾਲ ਤੰਦਰੁਸਤ ਹੋਣ ।”
(“’Physical Education should aim to provide the skilled leadership, adequate facilities and ample time. For affording full opportunity for individuals and groups to participate in situation that are physically wholesome, mentally stimulating and satisfying and socially sound.”) (J.F. Williams)
ਜੇ. ਆਰ. ਸ਼ਰਮਨ ਦੇ ਅਨੁਸਾਰ, ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਉਦੇਸ਼ ਲੋਕਾਂ ਦੇ ਅਨੁਭਵਾਂ ਨੂੰ ਇਸ ਸੀਮਾ ਤਕ ਪ੍ਰਭਾਵਿਤ ਕਰਨਾ ਹੈ ਕਿ ਹਰੇਕ ਵਿਅਕਤੀ ਆਪਣੀ ਸਮਰੱਥਾ ਅਨੁਸਾਰ ਸਮਾਜ ਵਿਚ ਠੀਕ ਤਰ੍ਹਾਂ ਰਹਿ ਸਕੇ, ਆਪਣੀਆਂ ਲੋੜਾਂ ਨੂੰ ਵਧਾ ਸਕੇ ਤੇ ਸੁਧਾਰ ਸਕੇ ਅਤੇ ਲੋਕਾਂ ਨੂੰ ਸੰਤੁਸ਼ਟ ਕਰਨ ਦੀ ਆਪਣੀ ਯੋਗਤਾ ਵਿਕਸਿਤ ਕਰ ਸਕੇ ।”
(“The aim of physical education is to influence the experiences of persons to the extent that each individual with in the limits of his capacity may be helped to adjust successfully in society, to increase and improve his wants and to develop the ability to satisfy his wants.”) (J.R. Sharman)
1. ਐੱਚ. ਕਲਰਕ (H. Clarke) ਨੇ ਉਦੇਸ਼ਾਂ ਨੂੰ ਨਿਮਨ ਤਿੰਨ ਭਾਗਾਂ ਵਿੱਚ ਵੰਡਿਆ ਹੈ
- ਸਰੀਰਕ ਤੰਦਰੁਸਤੀ ਵਿੱਚ ਵਾਧਾ,
- ਸਮਾਜਿਕ ਗੁਣਾਂ ਵਿੱਚ ਵਾਧਾ,
- ਸੰਸਕ੍ਰਿਤੀ ।
(According to H. Clarke mentions only three objectives of Physical education.)
- Physical fitness,
- Social efficiency,
- Culture.
2. ਹੇਰਿੰਗਟਨ (Hethrington) ਨੇ ਪੰਜ ਉਦੇਸ਼ ਦੱਸੇ ਹਨ-
- ਤੁਰੰਤ ਉਦੇਸ਼,
- ਦੂਰਵਰਤੀ ਉਦੇਸ਼,
- ਵਿਕਾਸਾਤਮਕ ਉਦੇਸ਼,
- ਸਮਾਜਿਕ ਸਤਰ ਉਦੇਸ਼,
- ਸਰੀਰਕ ਸਥਿਤੀ ਦਾ ਨਿਯੰਤਰਣ ।
(According to Hethrington classifies the five objectives of Physical education :
- Immediate objectives,
- Remote objectives,
- Development objectives,
- Social standard objectives,
- Objectives in control of health conditions.
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਸਲਾਹਕਾਰ ਬੋਰਡ ਦੇ ਵਿਚਾਰ (Views of Central Advisory Board of Physical Education-“ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਉਦੇਸ਼ ਹਰੇਕ ਬੱਚੇ ਨੂੰ ਸਰੀਰਕ, ਮਾਨਸਿਕ ਅਤੇ ਭਾਵਾਤਮਕ ਤੌਰ ‘ਤੇ ਯੋਗ ਬਣਾਉਣਾ ਹੈ ਅਤੇ ਉਸ ਵਿਚ ਇਸ ਤਰ੍ਹਾਂ ਦੇ ਨਿੱਜੀ ਅਤੇ ਸਮਾਜਿਕ ਗੁਣ ਵਿਕਸਿਤ ਕਰਨਾ ਹੈ ਜਿਸ ਨਾਲ ਉਹ ਸਮਾਜ ਦੇ ਦੂਜੇ ਮੈਂਬਰਾਂ ਦੇ ਨਾਲ ਪ੍ਰਸੰਨਤਾ ਪੂਰਵਕ ਰਹਿ ਸਕੇ ਅਤੇ ਚੰਗਾ ਨਾਗਰਿਕ ਬਣ ਸਕੇ ।”
(“The aim of physical education is to make every child physically, mentally and constitutionally fit and also to develop in him such personal and social qualities as will him live happily with others and build him up as a good citizen.”) | ਸਰੀਰਕ ਸਿੱਖਿਆ ਕਾਲਜਾਂ ਦੇ ਪ੍ਰਿੰਸੀਪਲਾਂ ਦੀ ਕਾਨਫਰੰਸ ਵਿਚ ਪ੍ਰਗਟ ਕੀਤੇ ਗਏ ਵਿਚਾਰ (Views expressed in conference of principals of Physical Training Colleges)-ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਉਦੇਸ਼ ਅਜਿਹੇ ਮੌਕੇ ਪੈਦਾ ਕਰਨਾ ਹੋਣਾ ਚਾਹੀਦਾ ਹੈ, ਜੋ ਕਿ ਭਾਰਤ ਦੇ ਬੱਚਿਆਂ ਤੇ ਨੌਜਵਾਨਾਂ ਦੇ ਤਨ, ਮਨ ਤੇ ਸਰੀਰਕ ਗਠਨ ਨੂੰ ਪ੍ਰਪੱਕ ਕਰਨ ਤੇ ਉਨ੍ਹਾਂ ਵਿਚ ਅਜਿਹੀਆਂ ਯੋਗਤਾਵਾਂ ਤੇ ਰੁਚੀਆਂ ਦਾ ਵਿਕਾਸ ਕਰਨ, ਜੋ ਕਿ ਬਦਲਦੇ ਸਮਾਜ ਵਿਚ ਉਨ੍ਹਾਂ ਦੇ ਲੰਮੇ ਤੇ ਰਚਨਾਤਮਕ ਜੀਵਨ ਲਈ ਸਹਾਈ ਹੋ ਸਕਣ ।”
(“Physical Education should aim to provide opportunities that will make the children and youth of India, Physically, mentally and constitutionally fit and develop in them the skills and attitudes conducive to long, happy and creating living in the changing society.”)
ਸਿੱਟਾ (Conclusion) – ਉਪਰੋਕਤ ਪਰਿਭਾਸ਼ਾਵਾਂ ਦੇ ਅਧਿਐਨ ਤੋਂ ਅਸੀਂ ਇਸ ਸਿੱਟੇ ਤੇ ਪੁੱਜਦੇ ਹਾਂ ਕਿ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਟੀਚਾ ਮਨੁੱਖ ਦਾ ਪੂਰਨ ਵਿਕਾਸ ਕਰਨਾ ਹੈ । ਲਗਪਗ ਸਾਰੇ ਵਿਦਵਾਨ ਇਸ ਵਿਚਾਰ ਨਾਲ ਸਹਿਮਤ ਹਨ ਕਿ
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਮਾਧਿਅਮ ਨਾਲ ਮਨੁੱਖਾਂ ਵਿਚ ਇਸ ਤਰ੍ਹਾਂ ਦੇ ਗੁਣ ਵਿਕਸਿਤ ਕੀਤੇ ਜਾਣ ਜਿਨ੍ਹਾਂ ਨਾਲ ਉਹਨਾਂ ਦਾ ਸਰੀਰਕ, ਮਾਨਸਿਕ ਅਤੇ ਭਾਵਾਤਮਕ ਵਿਕਾਸ ਹੋ ਸਕੇ ।
![]()
ਪ੍ਰਸ਼ਨ 4.
ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਦੇ ਕੋਈ ਤਿੰਨ ਉਦੇਸ਼ਾਂ ਦੀ ਵਿਸਥਾਰਪੂਰਵਕ ਜਾਣਕਾਰੀ ਦਿਓ । (Write the objectives of Physical Education in detail.)
ਉੱਤਰ-
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਉਦੇਸ਼ (Objectives of Physical Education)
ਜਿਵੇਂ ਪਹਿਲਾਂ ਦੱਸਿਆ ਜਾ ਚੁੱਕਾ ਹੈ ਕਿ ਟੀਚਾ (Aim) ਅੰਤਿਮ ਨਿਸ਼ਾਨਾ ਹੁੰਦਾ ਹੈ, ਜਿਸ ਦੀ ਪ੍ਰਾਪਤੀ ਲਈ ਕੁਝ ਉਦੇਸ਼ (Objectives) ਹੁੰਦੇ ਹਨ | ਆਮ ਤੌਰ ‘ਤੇ ਟੀਚਾ ਇਕ ਹੁੰਦਾ ਹੈ ਪਰ ਉਸ ਨੂੰ ਪ੍ਰਾਪਤ ਕਰਨ ਲਈ ਉਦੇਸ਼ ਅਨੇਕਾਂ ਹੋ ਸਕਦੇ ਹਨ । ਇਸੇ ਤਰ੍ਹਾਂ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਟੀਚਾ ਤਾਂ ਇਕ ਹੈ ਅਤੇ ਉਹ ਹੈ ਵਿਅਕਤੀ ਦਾ ਪੂਰਨ ਵਿਕਾਸ, ਪਰ ਇਸ ਟੀਚੇ ਦੀ ਪ੍ਰਾਪਤੀ ਲਈ ਕਈ ਉਦੇਸ਼ ਹਨ | ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਉਦੇਸ਼ ਸੰਬੰਧੀ ਵੱਖ-ਵੱਖ ਵਿਦਵਾਨਾਂ ਨੇ ਆਪਣੇ-ਆਪਣੇ ਵਿਚਾਰ ਪੇਸ਼ ਕੀਤੇ ਹਨ । ਪ੍ਰਮੁੱਖ ਵਿਦਵਾਨਾਂ ਦੇ ਮੱਤ ਹੇਠਾਂ ਦਿੱਤੇ ਜਾਂਦੇ ਹਨ
1. ਲਾਸਕੀ (Laski) ਅਨੁਸਾਰ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਹੇਠ ਲਿਖੇ ਪੰਜ ਉਦੇਸ਼ ਹਨ-
- ਸਰੀਰਕ ਪੱਖ ਵਾਲਾ ਵਿਕਾਸ (Physical Aspect of Development)
- ਭਾਵਾਤਮਕ ਪੱਖ ਵਾਲਾ ਵਿਕਾਸ (Emotional Aspect of Development
- ਸਮਾਜਿਕ ਪੱਖ ਵਾਲਾ ਵਿਕਾਸ (Social Aspect of Development)
- ਬੌਧਿਕ ਪੱਖ ਵਾਲਾ ਵਿਕਾਸ (Intellectual Aspect of Development)
- ਨਿਊਰੋ ਮਾਸਪੇਸ਼ੀ ਪੱਖ ਵਾਲਾ ਵਿਕਾਸ (Neuro-muscular Aspect of Development) ।
2. ਜੇ. ਬੀ. ਨੈਸ਼ (J.B. Nash) ਨੇ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਹੇਠ ਲਿਖੇ ਚਾਰ ਉਦੇਸ਼ਾਂ ਦਾ ਵਰਣਨ ਕੀਤਾ ਹੈ
- ਨਿਊਰੋ ਮਾਸਪੇਸ਼ੀ ਵਿਕਾਸ (Neuro-muscular Development)
- ਭਾਵਾਤਮਕ ਵਿਕਾਸ (Emotional Development)
- ਠੀਕ ਗੱਲ ਸਮਝਣ ਦੀ ਯੋਗਤਾ ਦਾ ਵਿਕਾਸ (Interpretative Development)
- ਸਰੀਰਕ ਅੰਗਾਂ ਦਾ ਵਿਕਾਸ (Organic Development) ।
3. ਇਕ ਦੂਜੇ ਵਿਦਵਾਨ ਬੁੱਕ ਵਾਲਟਰ (Buck Walter) ਨੇ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਉਦੇਸ਼ਾਂ ਨੂੰ ਤਿੰਨ ਵਰਗਾਂ ਵਿਚ | ਵੰਡਿਆ ਹੈ । ਇਹ ਵਰਗ ਹਨ-
- ਸਿਹਤ (Health)
- ਨੈਤਿਕ ਆਚਰਨ (Ethical Character) ।
- ਫਾਲਤੂ ਵਿਹਲੇ ਸਮੇਂ ਦੀ ਉੱਚਿਤ ਵਰਤੋਂ (Worthy Use of Leisure) ।
4. ਪ੍ਰਸਿੱਧ ਵਿਦਵਾਨ ਐੱਚ. ਸੀ. ਬੱਕ (H.C. Buck) ਨੇ ਸਰੀਰਕ ਸਿੱਖਿਆ ਉਦੇਸ਼ਾਂ ਦਾ ਵਰਗੀਕਰਨ ਇਸ ਤਰ੍ਹਾਂ ਕੀਤਾ ਹੈ-
- ਸਰੀਰਕ ਅੰਗਾਂ ਦਾ ਵਿਕਾਸ (Organic Development)
- ਨਿਊਰੋ ਮਾਸ-ਪੇਸ਼ੀਆਂ ਵਿਚ ਤਾਲਮੇਲ ਦਾ ਵਿਕਾਸ (Development of Neuro-muscular Co-ordination)
- ਖੇਡ ਅਤੇ ਸਰੀਰਕ ਕਿਰਿਆਵਾਂ ਪ੍ਰਤੀ ਉੱਚਿਤ ਦ੍ਰਿਸ਼ਟੀਕੋਣ ਦਾ ਵਿਕਾਸ (Development of right attitude towards play and physical activities)
- ਉੱਚਿਤ ਸਮਾਜਿਕ ਦ੍ਰਿਸ਼ਟੀਕੋਣ ਅਤੇ ਆਚਰਣ ਦਾ ਵਿਕਾਸ (Development of right social attitude and conduct)
- ਉੱਚਿਤ ਸਿਹਤ ਸੰਬੰਧੀ ਆਦਤਾਂ ਦਾ ਵਿਕਾਸ (Development of correct health habits) ।
ਇਸ ਤਰ੍ਹਾਂ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਕਈ ਹੋਰ ਵਿਦਵਾਨਾਂ ਨੇ ਵੀ ਆਪਣੇ-ਆਪਣੇ ਦ੍ਰਿਸ਼ਟੀਕੋਣ ਨਾਲ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਉਦੇਸ਼ਾਂ ਦਾ ਵਰਣਨ ਕੀਤਾ ਹੈ । ਇਹਨਾਂ ਵਿਚੋਂ ਐੱਚ. ਕਲਾਰਕ (H. Clark), ਹੈਥੇਰਿੰਗਟਨ (Hetherington), ਵੁੱਡ (Wood) ਅਤੇ ਕੈਸਿਡੀ (Cassidy) ਆਦਿ ਵਿਦਵਾਨਾਂ ਦੇ ਨਾਂ ਵਰਣਨਯੋਗ ਹਨ |
ਸਿੱਟਾ (Conclusion – ਇਹਨਾਂ ਸਾਰੇ ਵਿਦਵਾਨਾਂ ਦੇ ਵਿਚਾਰ ਦਾ ਅਧਿਐਨ ਕਰਨ ਨਾਲ ਇੱਕ ਗੱਲ ਬਿਲਕੁਲ ਸਪੱਸ਼ਟ ਹੈ । ਉਹ ਇਹ ਹੈ ਕਿ ਸਾਰੇ ਵਿਦਵਾਨ ਇਸ ਗੱਲ ਨਾਲ ਸਹਿਮਤ ਹਨ ਕਿ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਹੇਠ ਲਿਖੇ ਚਾਰ ਉਦੇਸ਼ ਹਨ-
- ਸਰੀਰਕ ਵਿਕਾਸ ਦੇ ਉਦੇਸ਼ (Physical development Objectives)
- ਮਾਨਸਿਕ ਵਿਕਾਸ ਦੇ ਉਦੇਸ਼ (Mental development Objectives)
- ਹਰਕਤ ਅਤੇ ਕਾਰਜ-ਸ਼ਕਤੀ ਦੇ ਵਿਕਾਸ ਦੇ ਉਦੇਸ਼ (Motor development Objectives)
- ਸਮਾਜਿਕ ਵਿਕਾਸ ਦੇ ਉਦੇਸ਼ (Social development Objectives) ।
ਆਓ, ਇਹਨਾਂ ਸਾਰੇ ਉਦੇਸ਼ਾਂ ਦਾ ਵਾਰੋ-ਵਾਰੀ ਵਰਣਨ ਕਰੀਏ-
1. ਸਰੀਰਕ ਵਿਕਾਸ ਦੇ ਉਦੇਸ਼ (Physical development Objectives) – ਇਹਨਾਂ ਉਦੇਸ਼ਾਂ ਅਧੀਨ ਉਹ ਉਦੇਸ਼
ਸ਼ਾਮਿਲ ਕੀਤੇ ਜਾਂਦੇ ਹਨ ਜਿਨ੍ਹਾਂ ਦੁਆਰਾ ਮਨੁੱਖ ਆਪਣੇ ਸਰੀਰ ਨੂੰ ਸ਼ਕਤੀਸ਼ਾਲੀ, ਸੁਡੌਲ, ਦਿਲਖਿੱਚਵਾਂ ਅਤੇ ਤੰਦਰੁਸਤ ਬਣਾ ਕੇ ਆਪਣਾ ਸਰੀਰਕ ਵਿਕਾਸ ਕਰਦਾ ਹੈ । ਇਹਨਾਂ ਉਦੇਸ਼ਾਂ ਦੀ ਪ੍ਰਾਪਤੀ ਲਈ ਉਹ ਕਸਰਤ ਕਰਦਾ ਹੈ ਅਤੇ ਖੇਡਾਂ ਵਿਚ ਸਰਗਰਮ ਭਾਗ (Active Part) ਲੈਂਦਾ ਹੈ ।
2. ਮਾਨਸਿਕ ਵਿਕਾਸ ਦੇ ਉਦੇਸ਼ (Mental development Objectives) – ਮਾਨਸਿਕ ਵਿਕਾਸ ਦੇ ਉਦੇਸ਼ਾਂ ਵਿਚ ਉਹ ਉਦੇਸ਼ ਆ ਜਾਂਦੇ ਹਨ ਜਿਨ੍ਹਾਂ ਦੁਆਰਾ ਬੱਚਿਆਂ ਦੇ ਮਾਨਸਿਕ ਤਨਾਅ ਅਤੇ ਦਬਾਅ ਨੂੰ ਦੂਰ ਭਜਾਇਆ ਜਾਂਦਾ ਹੈ । ਉਹਨਾਂ ਨੂੰ ਉੱਚਿਤ ਤਰ੍ਹਾਂ ਨਾਲ ਸੋਚਣ ਦੇ ਢੰਗ ਦੀ ਸਿੱਖਿਆ ਦਿੱਤੀ ਜਾਂਦੀ ਹੈ । ਇਸ ਤੋਂ ਇਲਾਵਾ ਉਹਨਾਂ
ਨੂੰ ਵੱਖ-ਵੱਖ ਔਕੜਾਂ ‘ਤੇ ਕਾਬੂ ਪਾਉਣਾ ਅਤੇ ਸਮੱਸਿਆ ਦਾ ਹੱਲ ਕਰਨ ਦੀ ਸਿਖਲਾਈ ਦਿੱਤੀ ਜਾਂਦੀ ਹੈ ।
3. ਹਰਕਤ ਅਤੇ ਕਾਰਜ-ਸ਼ਕਤੀ ਦੇ ਵਿਕਾਸ ਦੇ ਉਦੇਸ਼ (Motor development Objectives) – ਇਹਨਾਂ ਉਦੇਸ਼ਾਂ | ਅਧੀਨ ਉਹਨਾਂ ਉਦੇਸ਼ਾਂ ਦੀ ਗਿਣਤੀ ਕੀਤੀ ਜਾਂਦੀ ਹੈ ਜਿਨ੍ਹਾਂ ਦੁਆਰਾ ਮਨੁੱਖ ਆਪਣੀਆਂ ਸਰੀਰਕ ਕਿਰਿਆਵਾਂ ਬਿਨਾਂ ਜ਼ਿਆਦਾ ਬਲ ਲਗਾਏ ਸੌਖ ਨਾਲ ਚੰਗੀ ਤਰ੍ਹਾਂ ਕਰ ਸਕਦਾ ਹੈ ।
4. ਸਮਾਜਿਕ ਵਿਕਾਸ ਦੇ ਉਦੇਸ਼ (Social development Objectives) – ਇਹਨਾਂ ਉਦੇਸ਼ਾਂ ਵਿਚ ਉਹ ਉਦੇਸ਼ ਸ਼ਾਮਿਲ ਹਨ ਜਿਨ੍ਹਾਂ ਦੁਆਰਾ ਇਕ ਵਿਅਕਤੀ ਵਿਚ ਅਗਵਾਈ (Leadership). ਸਹਿਣਸ਼ੀਲਤਾ (Tolerance), ਸਹਿਯੋਗ (Co-operation), ਨਿਡਰਤਾ (Boldness), ਆਤਮ-ਸੰਜਮ (Self-Discipline), ਸ਼ੈ-ਪ੍ਰਗਟਾਵੇ (SelfExpression) ਆਦਿ ਗੁਣ ਵਿਕਸਿਤ ਕੀਤੇ ਜਾਂਦੇ ਹਨ ਜਿਨ੍ਹਾਂ ਨੂੰ ਪ੍ਰਾਪਤ ਕਰਕੇ ਉਹ ਇਕ ਆਦਰਸ਼ ਨਾਗਰਿਕ
ਅਤੇ ਸਮਾਜ ਦਾ ਉਪਯੋਗੀ ਮੈਂਬਰ ਬਣ ਸਕਦਾ ਹੈ ।
![]()
ਪ੍ਰਸ਼ਨ 5.
ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਦਾ ਕੀ ਮਹੱਤਵ ਹੈ ? ਇਸ ਦੀ ਵਿਸਥਾਰਪੂਰਵਕ ਜਾਣਕਾਰੀ ਦਿਓ । (Write the importance of Physical Education in detail.)
ਉੱਤਰ-
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਮਹੱਤਵ (Importance of Physical Education)
1. ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਪਾਠ-ਕ੍ਰਮ (Curriculum of Physical Education) – ਇਹ ਮੰਨ ਲਿਆ ਗਿਆ ਹੈ। ਕਿ ਸਰੀਰਕ ਸਿੱਖਿਆ ਸਾਧਾਰਨ ਸਿੱਖਿਆ ਦਾ ਹੀ ਇਕ ਅੰਗ ਹੈ । ਸਰੀਰਕ ਸਿੱਖਿਆ ਰਾਹੀਂ ਮਨੁੱਖ ਵਿਚ ਬਹੁਤ ਸਾਰੇ ਗੁਣ ਪੈਦਾ ਕੀਤੇ ਜਾ ਸਕਦੇ ਹਨ, ਜਿਹੜੇ ਕਿ ਰਾਸ਼ਟਰੀ ਏਕਤਾ ਲਈ ਬੜੇ ਜ਼ਰੂਰੀ ਹਨ । ਸਰੀਰਕ ਸਿੱਖਿਆ। ਦੇ ਪਾਠ-ਕ੍ਰਮ ਰਾਹੀਂ ਮਨੁੱਖ ਵਿਚ ਸਹਿਣਸ਼ੀਲਤਾ, ਸਮਾਜਿਕਤਾ, ਨਾਗਰਿਕਤਾ ਅਤੇ ਦੂਜਿਆਂ ਲਈ ਸਤਿਕਾਰ ਦੀ ਭਾਵਨਾ ਸਿਖਾਈ ਜਾ ਸਕਦੀ ਹੈ | ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਕਾਰਜ-ਕੂਮਾਂ ਵਿਚ ਕਿਸੇ ਤਰ੍ਹਾਂ ਦਾ ਭੇਦ-ਭਾਵ ਨਹੀਂ ਰੱਖਿਆ ਜਾਂਦਾ ਹੈ, ਇਸ ਤਰ੍ਹਾਂ ਇਹ ਰਾਸ਼ਟਰੀ ਏਕਤਾ ਕਾਇਮ ਕਰਨ ਵਿਚ ਪੂਰਾ ਹਿੱਸਾ ਪਾਉਂਦੀ ਹੈ ।
2. ਸਰੀਰਕ ਸਿੱਖਿਆ ਵਿਚ ਫ਼ਿਰਕੂਪੁਣੇ ਲਈ ਕੋਈ ਸਥਾਨ ਨਹੀਂ (No place for communalism in Physical Education) – ਸਰੀਰਕ ਸਿੱਖਿਆ ਕਿਸੇ ਤਰ੍ਹਾਂ ਦੇ ਫ਼ਿਰਕੂਪੁਣੇ ਨੂੰ ਆਪਣੇ ਅੰਦਰ ਨਹੀਂ ਆਉਣ ਦਿੰਦੀ । ਸਰੀਰਕ ਸਿੱਖਿਆ ਰੰਗ, ਨਸਲ, ਜਾਤ-ਪਾਤ, ਧਰਮ ਆਦਿ ਦੇ ਭੇਦ-ਭਾਵਾਂ ਨੂੰ ਸਵੀਕਾਰ ਨਹੀਂ ਕਰਦੀ । ਇਸ ਦੇ ਸਾਹਮਣੇ ਸਮੁੱਚੇ ਮਨੁੱਖ ਦੀ ਭਲਾਈ ਦਾ ਹੀ ਉਦੇਸ਼ ਹੁੰਦਾ ਹੈ । ਇਹ ਅਜਿਹੇ ਤੰਗ ਵਿਚਾਰਾਂ ਨੂੰ ਨਹੀਂ ਸਹਾਰਦੀ, ਜਿਨ੍ਹਾਂ ਤੋਂ ਫ਼ਿਰਕੂ ਝਗੜੇ ਪੈਦਾ ਹੋਣ | ਫ਼ਿਰਕੂਪੁਣਾ ਸਾਡੇ ਦੇਸ਼ ਲਈ ਭਾਰੀ ਖ਼ਤਰਾ ਹੈ । ਸਰੀਰਕ ਸਿੱਖਿਆ ਇਸ ਖ਼ਤਰੇ ਨੂੰ ਦੂਰ ਕਰਕੇ ਰਾਸ਼ਟਰੀ ਏਕਤਾ ਵਿਚ ਵਾਧਾ ਕਰਦੀ ਹੈ ।
3. ਨਾ ਬਰਾਬਰੀ ਅਤੇ ਸਰੀਰਕ ਸਿੱਖਿਆ (Inequality and Physical Education) – ਸਰੀਰਕ ਸਿੱਖਿਆ ਕਿਸੇ ਪ੍ਰਕਾਰ ਦੀ ਨਾ-ਬਰਾਬਰੀ (Inequality) ਨੂੰ ਸਵੀਕਾਰ ਨਹੀਂ ਕਰਦੀ । ਇਸ ਲਈ ਅਮੀਰ-ਗ਼ਰੀਬ, ਛੋਟਾ-ਵੱਡਾ ਸਭ ਕੋਈ ਬਰਾਬਰ ਹਨ | ਸਭ ਲੋਕ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਕਾਰਜ-ਕੂਮ ਵਿਚ ਇੱਕੋ ਜਿਹੇ ਹਿੱਸੇਦਾਰ ਹੁੰਦੇ ਹਨ | ਅੱਜ ਦੇ ਯੁੱਗ ਵਿਚ ਨਾ-ਬਰਾਬਰੀ ਇਕ ਭਾਰੀ ਸਮੱਸਿਆ ਹੈ | ਸਰੀਰਕ ਸਿੱਖਿਆ ਇਸ ਸਮੱਸਿਆ ਦਾ ਇਲਾਜ ਕਰਕੇ ਸਭ ਮਨੁੱਖਾਂ ਅੰਦਰ ਰਾਸ਼ਟਰੀ ਏਕਤਾ ਦੀ ਭਾਵਨਾ ਭਰਪੂਰ ਕਰਦੀ ਹੈ ।
4. ਸਰੀਰਕ ਸਿੱਖਿਆ ਅਤੇ ਪ੍ਰਾਂਤਵਾਦ (Provincialism and Physical Education) – ਸਰੀਰਕ ਸਿੱਖਿਆ ਵਿਚ ਪ੍ਰਾਂਤਵਾਦ ਦਾ ਕੋਈ ਮਹੱਤਵ ਨਹੀਂ ਹੈ । ਜਦੋਂ ਕੋਈ ਖਿਡਾਰੀ ਸਰੀਰਕ ਕਿਰਿਆ ਕਰਦਾ ਹੈ, ਤਾਂ ਉਸ ਵਿਚ ਇਸ ਤਰ੍ਹਾਂ ਦੀ ਕੋਈ ਭਾਵਨਾ ਨਹੀਂ ਹੁੰਦੀ ਕਿ ਉਹ ਇਸ ਪ੍ਰਾਂਤ ਜਾਂ ਉਸ ਪ੍ਰਾਂਤ ਦਾ ਵਸਨੀਕ ਹੈ ।ਉਸਦੇ ਸਾਹਮਣੇ ਮਾਨਵ ਭਲਾਈ ਦਾ ਇੱਕੋ ਇਕ ਉਦੇਸ਼ ਹੁੰਦਾ ਹੈ । ਸਭ ਮਿਲ-ਜੁਲ ਕੇ ਖੇਡਾਂ ਖੇਡਦੇ ਹਨ ਅਤੇ ਇਕ ਦੂਜੇ ਦੀਆਂ ਭਾਵਨਾਵਾਂ ਦਾ ਸਤਿਕਾਰ ਕਰਦੇ ਹਨ । ਇਸ ਲਈ ਉਨ੍ਹਾਂ ਵਿਚ ਏਕਤਾ ਵੱਧਦੀ ਹੈ ਅਤੇ ਉਹ ਸਭ ਮਿਲ ਕੇ ਦੇਸ਼ ਨੂੰ ਹੋਰ ਸ਼ਕਤੀਸ਼ਾਲੀ ਬਣਾਉਂਦੇ ਹਨ ਅਤੇ ਰਾਸ਼ਟਰੀ ਏਕਤਾ ਵਿਚ ਵਾਧਾ ਕਰਦੇ ਹਨ ।
5. ਭਾਸ਼ਾ-ਵਾਦ ਅਤੇ ਸਰੀਰਕ ਸਿੱਖਿਆ (Linguism and Physical Education) – ਜਿਸ ਦੇਸ਼ ਵਿਚ ਭਾਸ਼ਾ-ਵਿਵਾਦ ਬਹੁਤ ਜ਼ੋਰ ਫੜ ਲੈਂਦਾ ਹੈ, ਉਸ ਦੇਸ਼ ਵਿਚ ਰਾਸ਼ਟਰੀ ਏਕਤਾ ਬਹੁਤ ਸਮੇਂ ਤਕ ਕਾਇਮ ਨਹੀਂ ਰਹਿ ਸਕਦੀ | ਭਾਰਤ ਦੇਸ਼ ਵਿਚ ਬਹੁਤ ਸਾਰੀਆਂ ਭਾਸ਼ਾਵਾਂ ਬੋਲੀਆਂ ਜਾਂਦੀਆਂ ਹਨ । ਕਈ ਇਲਾਕਿਆਂ ਵਿਚ ਭਾਸ਼ਾ ਦੇ ਨਾਂ ਉੱਤੇ ਲੜਾਈ ਝਗੜੇ ਵੀ ਹੁੰਦੇ ਹਨ । ਕਿਤੇ ਪੰਜਾਬੀ, ਕਿਤੇ ਤਾਮਿਲ ਭਾਸ਼ਾ ਦਾ ਝਗੜਾ ਹੈ, ਤਾਂ ਕਿਤੇ ਬੰਗਾਲੀ ਅਤੇ ਉੜੀਆ ਦਾ । ਇਕ ਥਾਂ ਦੀ ਭਾਸ਼ਾ ਦੂਜੀ ਥਾਂ ਉੱਤੇ ਸਮਝਣੀ ਮੁਸ਼ਕਲ ਹੈ | ਪਰ ਸਰੀਰਕ ਸਿੱਖਿਆ ਕਿਸੇ ਵੀ ਭਾਸ਼ਾਈ ਵਿਤਕਰੇ ਨੂੰ ਪਰਵਾਨ ਨਹੀਂ ਕਰਦੀ । ਖਿਡਾਰੀ ਭਾਵੇਂ ਉਹ ਬੰਗਾਲੀ ਬੋਲਦਾ ਹੈ ਭਾਵੇਂ ਪੰਜਾਬੀ, ਹਰੇਕ ਖਿਡਾਰੀ ਨੂੰ ਆਪਣਾ ਭਰਾ ਸਮਝਦਾ ਹੈ | ਸਭ ਖਿਡਾਰੀ ਇਕ ਟੀਮ ਦੇ ਰੂਪ ਵਿਚ ਖੇਡ-ਮੈਦਾਨ ਵਿਚ ਆਉਂਦੇ ਹਨ ਅਤੇ ਪ੍ਰਸਪਰ ਮਿਲ-ਜੁਲ ਕੇ ਦੇਸ਼ ਦੀ ਸ਼ਾਨ ਵਧਾਉਣ ਦਾ ਯਤਨ ਕਰਦੇ ਹਨ । ਇਸ ਤਰ੍ਹਾਂ ਸਰੀਰਕ ਸਿੱਖਿਆ ਭਾਸ਼ਾਵਾਦ ਨੂੰ ਘਟਾਉਣ ਅਤੇ ਰਾਸ਼ਟਰੀ ਏਕਤਾ ਵਿਚ ਵਾਧਾ ਕਰਨ ਦਾ ਯਤਨ ਕਰਦੀ ਹੈ ।
6. ਵਿਹਲਾ ਸਮਾਂ ਅਤੇ ਸਰੀਰਕ ਸਿੱਖਿਆ (Leisure time and Physical Education) – ਵਿਹਲਾ ਸਮਾਂ ਉਹ ਹੁੰਦਾ ਹੈ, ਜਦ ਮਨੁੱਖ ਕੋਲ ਕਰਨ ਨੂੰ ਕੋਈ ਕੰਮ ਨਾ ਹੋਵੇ । ਕਈ ਲੋਕ ਵਿਹਲੇ ਸਮੇਂ ਦਾ ਕੋਈ ਲਾਭ ਨਹੀਂ ਉਠਾਉਂਦੇ, ਸਗੋਂ ਇਸ ਸਮੇਂ ਵਿਅਰਥ ਝਗੜੇ, ਬਹਿਸ ਅਤੇ ਲੜਾਈ ਆਦਿ ਵਿਚ ਪੈ ਕੇ ਆਪਣਾ ਅਤੇ ਦੂਜਿਆਂ ਦਾ ਨੁਕਸਾਨ ਕਰਦੇ ਹਨ, ਜਿਸ ਨਾਲ ਦੇਸ਼ ਦੇ ਸਾਹਮਣੇ ਕਈ ਸਮੱਸਿਆਵਾਂ ਖੜ੍ਹੀਆਂ ਹੋ ਜਾਂਦੀਆਂ ਹਨ ਅਤੇ ਉਹ ਕਈ ਉਲਝਣਾਂ ਵਿਚ ਫਸ ਜਾਂਦਾ ਹੈ । ਕਈ ਵਾਰ ਇਹ ਝਗੜੇ ਇੰਨੇ ਵੱਧ ਜਾਂਦੇ ਹਨ ਕਿ ਦੇਸ਼ ਦੀ ਏਕਤਾ ਨੂੰ ਵੀ ਖ਼ਤਰਾ ਪੈਦਾ ਹੋ ਜਾਂਦਾ ਹੈ । ਵਿਹਲੇ ਸਮੇਂ ਦੀ ਯੋਗ ਵਰਤੋਂ ਕਰਾਉਣ ਲਈ ਸਰੀਰਕ ਸਿੱਖਿਆ ਬੜਾ ਚੰਗਾ ਪ੍ਰੋਗਰਾਮ ਪੇਸ਼ ਕਰਦੀ ਹੈ । ਇਨ੍ਹਾਂ ਪ੍ਰੋਗਰਾਮਾਂ ਵਿਚ ਸਭ ਬੱਚਿਆਂ ਅਤੇ ਨੌਜਵਾਨਾਂ ਦੇ ਵਿਹਲੇ ਸਮੇਂ ਦੀ ਚੰਗੀ ਵਰਤੋਂ ਹੁੰਦੀ ਹੈ ਅਤੇ ਉਨ੍ਹਾਂ ਦੀ ਸ਼ਕਤੀ ਠੀਕ ਪਾਸੇ ਲੱਗਦੀ ਹੈ । ਇਸ ਤਰ੍ਹਾਂ ਰਾਸ਼ਟਰੀ ਏਕਤਾ ਦੀ ਸਮੱਸਿਆ ਹੱਲ ਹੋ ਜਾਂਦੀ ਹੈ ।
7. ਦੇਸ਼-ਭਗਤੀ, ਅਨੁਸ਼ਾਸਨ ਅਤੇ ਸਹਿਣਸ਼ੀਲਤਾ (Patriotism, Discipline and Toleration) – ਸਰੀਰਕ ਸਿੱਖਿਆ ਰਾਹੀਂ, ਦੇਸ਼ ਭਗਤੀ ਦੀ ਭਾਵਨਾ ਪੈਦਾ ਹੁੰਦੀ ਹੈ । ਸਰੀਰਕ ਸਿੱਖਿਆ ਨੌਜਵਾਨਾਂ ਵਿਚ ਦੇਸ਼ ਭਗਤੀ ਦੀ ਭਾਵਨਾ ਉਤਪੰਨ ਕਰਕੇ ਉਸ ਨੂੰ ਚੰਗੀ ਤਰ੍ਹਾਂ ਉਸਾਰਦੀ ਹੈ । N.C.C., A.C.C., Scouting, Girl Guiding · ਅਤੇ N.S.S. ਰਾਹੀਂ ਨੌਜਵਾਨਾਂ ਨੂੰ ਜਿੱਥੇ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਕੇ ਬੜੇ ਸੁੰਦਰੂ ਢੰਗ ਨਾਲ ਵਿਕਸਿਤ ਕੀਤਾ ਜਾਂਦਾ ਹੈ, ਉੱਥੇ ਉਨ੍ਹਾਂ ਅੰਦਰ ਦੇਸ਼ ਭਗਤੀ ਅਤੇ ਦੇਸ਼ ਪ੍ਰੇਮ ਦੀ ਭਾਵਨਾ ਵੀ ਪੈਦਾ ਕੀਤੀ ਜਾਂਦੀ ਹੈ । ਖੇਡ ਦੇ ਮੈਦਾਨ ਵਿਚੋਂ ਖਿਡਾਰੀ ਅਨੁਸ਼ਾਸਨ ਅਤੇ ਕਰਤੱਵ ਪਾਲਣ ਦੇ ਗੁਣ ਵੀ ਸਿੱਖਦੇ ਹਨ ਅਤੇ ਆਪਣੀ ਸਹਿਣਸ਼ੀਲਤਾ ਦੀ ਭਾਵਨਾ ਵਿਚ ਵਾਧਾ ਕਰਦੇ ਹਨ | ਸਰੀਰਕ ਸਿੱਖਿਆ ਇਨ੍ਹਾਂ ਗੁਣਾਂ ਨੂੰ ਵੱਧ ਤੋਂ ਵੱਧ ਮਹੱਤਤਾ ਦੇ ਕੇ ਨੌਜਵਾਨਾਂ ਦੀ ਠੀਕ ਅਗਵਾਈ ਕਰਦੀ ਹੈ ਅਤੇ ਉਹਨਾਂ ਨੂੰ ਰਾਸ਼ਟਰੀ ਏਕਤਾ ਵਿਚ ਵਾਧਾ ਕਰਨ ਦੀ ਜਾਚ ਸਿਖਾਉਂਦੀ ਹੈ ।
8. ਰਾਸ਼ਟਰੀ ਆਚਰਨ-ਉਸਾਰੀ ਅਤੇ ਸਰੀਰਕ ਸਿੱਖਿਆ (National Character and Physical Education| ਲੋਕਾਂ ਅੰਦਰ ਰਾਸ਼ਟਰੀ ਏਕਤਾ ਦੀ ਭਾਵਨਾ ਜਗਾਉਣ ਅਤੇ ਉਨ੍ਹਾਂ ਅੰਦਰ ਰਾਸ਼ਟਰੀ ਆਚਰਨ ਦੀ ਉਸਾਰੀ ਕਰਨ ਵਿਚ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਬਹੁਤ ਵੱਡਾ ਹਿੱਸਾ ਹੈ । ਜੇ ਦੇਸ਼ ਦੇ ਲੋਕਾਂ ਵਿਚ ਰਾਸ਼ਟਰੀ ਆਚਰਨ ਦੀ ਕਮੀ ਹੋਵੇ ਤਾਂ ਦੇਸ਼ ਦਾ ਕੋਈ ਵੀ ਕੰਮ ਠੀਕ ਤਰ੍ਹਾਂ ਨਹੀਂ ਚਲ ਸਕਦਾ ਅਤੇ ਦੇਸ਼ ਕੋਈ ਉੱਨਤੀ ਨਹੀਂ ਕਰ ਸਕਦਾ । ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਪ੍ਰੋਗਰਾਮ ਇਸ ਤਰ੍ਹਾਂ ਬਣਾਏ ਜਾਂਦੇ ਹਨ ਕਿ ਨੌਜਵਾਨਾਂ ਅੰਦਰ ਦੇਸ਼ ਪ੍ਰੇਮ ਅਤੇ ਦੇਸ਼ ਸਤਿਕਾਰ ਦੀ ਭਾਵਨਾ ਦੇ ਨਾਲ ਨਾਲ ਰਾਸ਼ਟਰੀ ਆਚਰਨ ਦੀ ਉਸਾਰੀ ਹੁੰਦੀ ਹੈ । ਇਹ ਰਾਸ਼ਟਰੀ ਆਚਰਨ ਰਾਸ਼ਟਰੀ ਏਕਤਾ ਨੂੰ ਕਾਇਮ ਰੱਖਣ ਵਿਚ ਬੜਾ ਭਾਰੀ ਹਿੱਸਾ ਪਾਉਂਦਾ ਹੈ । ਇਸ ਤੋਂ ਬਿਨਾਂ ਰਾਸ਼ਟਰੀ ਏਕਤਾ ਬਹੁਤ ਸਮਾਂ ਕਾਇਮ ਨਹੀਂ ਰਹਿ ਸਕਦੀ । ਸਰੀਰਕ ਸਿੱਖਿਆ ਰਾਸ਼ਟਰੀ ਆਚਰਨ ਰਾਹੀਂ ਇਸ ਏਕਤਾ ਨੂੰ ਸਦਾ ਲਈ ਕਾਇਮ ਰੱਖਦੀ ਹੈ ।
![]()
PSEB 11th Class Physical Education Guide ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਅਤੇ ਇਸ ਦੀ ਮਹੱਤਤਾ Important Questions and Answers
ਕੁੱਝ ਹੋਰ ਮਹੱਤਵਪੂਰਨ ਪ੍ਰਸ਼ਨ
ਵਸਤੂਨਿਸ਼ਠ ਪ੍ਰਸ਼ਨ (Objective Type Questions)
ਪ੍ਰਸ਼ਨ 1.
ਕੀ ਸਰੀਰਕ ਸਿੱਖਿਆ ਅਤੇ ਸਿਹਤ ਸਿੱਖਿਆ ਇੱਕੋ ਹੈ ?
ਉੱਤਰ-
ਨਹੀਂ, ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਅਤੇ ਸਿਹਤ ਸਿੱਖਿਆ ਇੱਕੋ ਨਹੀਂ ਹੈ ।
ਪ੍ਰਸ਼ਨ 2.
ਸਰੀਰਕ ਤੰਦਰੁਸਤੀ ਵਿੱਚ ਵਾਧਾ, ਸਮਾਜਿਕ ਗੁਣ ਵਿਚ ਵਾਧਾ, ਸੰਸਕ੍ਰਿਤੀ ‘ ਕਿਸ ਦਾ ਕਥਨ ਹੈ ?
(a) ਐੱਚ. ਕਲਰਕ
(b) ਹੇਥਰਿੰਗਟਨ
(c) ਬੁੱਕ ਵਾਲਟਰ
(d) ਜੇ. ਬੀ. ਨੈਸ਼ ।
ਉੱਤਰ-
(a) ਐੱਚ. ਕਲਰਕ ।
ਪ੍ਰਸ਼ਨ 3.
‘‘ਤੁਰੰਤ ਉਦੇਸ਼, ਦੁਰਵਰਤੀ ਉਦੇਸ਼, ਵਿਕਾਸਾਤਮਕ ਉਦੇਸ਼, ਸਮਾਜਿਕ ਸਤਰ ਉਦੇਸ਼, ਸਰੀਰਕ ਸਥਿਤੀ ਦਾ ਨਿਯੰਤਰਣ ।’ ਇਹ ਉਦੇਸ਼ ਕਿਸ ਦੇ ਅਨੁਸਾਰ ਹਨ ?
(a) ਜੀ. ਬੀ. ਨੈਸ਼ .
(b) ਹੇਰਿੰਗਟਨ
(c) ਐੱਚ. ਸੀ. ਬੱਕ
(d) ਲਾਂਸਕੀ ।
ਉੱਤਰ-
(b) ਹੇਥਰਿੰਗਟਨ ।
ਪ੍ਰਸ਼ਨ 4.
ਨਿਉਰੋ ਮਾਸਪੇਸ਼ੀ ਵਿਕਾਸ, ਭਾਵਾਤਮਕ ਵਿਕਾਸ, ਠੀਕ ਗੱਲ ਸਮਝਣ ਦੀ ਯੋਗਤਾ ਦਾ ਵਿਕਾਸ, ਸਰੀਰਕ ਅੰਗਾਂ ਦਾ ਵਿਕਾਸ ।’ ਇਹ ਉਦੇਸ਼ ਕਿਸ ਦੇ ਅਨੁਸਾਰ ਹਨ ?
(a) ਜੇ. ਬੀ. ਨੈਸ਼
(b) ਬੁੱਕ ਵਾਲਟਰ
(c) ਐੱਚ. ਸੀ. ਬੱਕ
(d) ਲਾਂਸਕੀ ।
ਉੱਤਰ-
(a) ਜੇ. ਬੀ. ਨੈਸ਼ ।
ਪ੍ਰਸ਼ਨ 5.
‘‘ਸਰੀਰਕ ਸਿੱਖਿਆ ਉਨ੍ਹਾਂ ਸਾਰਿਆਂ ਤਜਰਬਿਆਂ ਦਾ ਜੋੜ ਹੈ ਜੋ ਕਿਸੇ ਵਿਅਕਤੀ ਨੂੰ ਸਰੀਰਕ ਹਰਕਤ ਰਾਹੀਂ ਪ੍ਰਾਪਤ ਹੁੰਦੇ ਹਨ । ਇਹ ਕਥਨ ਕਿਸ ਦਾ ਹੈ ?
(a) ਡੀ ਉਬਰਟਿਊਫਰ
(b) ਆਰ. ਕੈਸਿਡੀ
(c) ਜੇ.ਬੀ. ਨੈਸ਼
d) ਜੇ. ਐੱਫ਼ ਵਿਲੀਅਮਜ਼ ।
ਉੱਤਰ-
(a) ਡੀ ਉਬਰਟਿਊਫਰ ।
ਪ੍ਰਸ਼ਨ 6.
ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਕੀ ਹੈ ?
ਉੱਤਰ-
ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਇੱਕ ਅਜਿਹਾ ਗਿਆਨ ਹੈ ਜੋ ਸਰੀਰ ਨਾਲ ਸੰਬੰਧ ਰੱਖਦਾ ਹੈ ।
![]()
ਪ੍ਰਸ਼ਨ 7.
‘‘ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਵਿਅਕਤੀ ਦੀਆਂ ਕੁੱਲ ਸਰੀਰਿਕ ਕਿਰਿਆਵਾਂ ਦਾ ਜੋੜ ਹੈ ਜਿਹੜੀਆਂ ਆਪਣੀ ਭਿੰਨਤਾ ਅਨੁਸਾਰ ਚੁਣੀਆਂ ਜਾਂਦੀਆਂ ਹਨ ਅਤੇ ਆਪਣੇ ਮੰਤਵ ਅਨੁਸਾਰ ਵਰਤੀਆਂ ਜਾਂਦੀਆਂ ਹਨ” ਕਿਸ ਦਾ ਕਥਨ ਹੈ ?
ਉੱਤਰ-
ਜੇ.ਐੱਫ਼. ਵਿਲੀਅਮਜ਼ ਦਾ ।
ਪ੍ਰਸ਼ਨ 8.
“ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਵਿੱਦਿਆ ਦੇ ਸਮੁੱਚੇ ਖੇਤਰ ਦਾ ਉਹ ਹਿੱਸਾ ਹੈ ਜੋ ਵੱਡੇ ਪੱਠਿਆਂ ਦੇ ਕਾਰਜਾਂ ਦੇ ਪ੍ਰਤੀ ਕਿਰਿਆਵਾਂ ਨਾਲ ਸੰਬੰਧ ਰੱਖਦਾ ਹੈ ।” ਕਿਸ ਦਾ ਕਥਨ ਹੈ ?
ਉੱਤਰ-
ਜੇ. ਬੀ. ਨੈਸ਼ ਦਾ ।
ਪ੍ਰਸ਼ਨ 9.
‘‘ਸਰੀਰਕ ਸਿੱਖਿਆ ਸਮੁੱਚੀ ਸਿੱਖਿਆ ਦੀ ਕਿਰਿਆ ਦਾ ਇਕ ਅਨਿੱਖੜਵਾਂ ਅੰਗ ਹੈ ਅਤੇ ਉੱਦਮ ਦੇ ਖੇਤਰ ਵਿਚ ਇਸ ਦਾ ਉਦੇਸ਼ ਸਰੀਰਕ, ਮਾਨਸਿਕ, ਭਾਵਾਤਮਕ ਅਤੇ ਸਮਾਜਿਕ ਰੂਪ ਤੋਂ ਸੰਪੂਰਨ ਨਾਗਰਿਕਾਂ ਦਾ ਅਜਿਹੀਆਂ ਸਰੀਰਕ ਕਿਰਿਆਵਾਂ ਦੇ ਮਾਧਿਅਮ ਰਾਹੀਂ ਵਿਕਾਸ ਕਰਨਾ ਜਿਨ੍ਹਾਂ ਦੀ ਚੋਣ ਇਨ੍ਹਾਂ ਉਦੇਸ਼ਾ ਨੂੰ ਸਾਹਮਣੇ ਰੱਖ ਕੇ ਕੀਤੀ ਜਾਏ ।’ ਕਿਸ ਦਾ ਕਥਨ ਹੈ ?
ਉੱਤਰ-
ਚਾਰਲਸ ਏ-ਬੂਚਰ ।
ਬਹੁਤ ਛੋਟੇ ਉੱਤਰਾਂ ਵਾਲੇ ਪ੍ਰਸ਼ਨ (Very Short Answer Type Questions)
ਪ੍ਰਸ਼ਨ 1.
ਸਰੀਰਿਕ ਸਿੱਖਿਆ ਦੇ ਕੋਈ ਤਿੰਨ ਉਦੇਸ਼ ਲਿਖੋ ।
ਉੱਤਰ-
- ਸਰੀਰਿਕ ਵਿਕਾਸ,
- ਮਾਨਸਿਕ ਵਿਕਾਸ,
- ਭਾਵਨਾਤਮਿਕ ਵਿਕਾਸ,
- ਸਮਾਜਿਕ ਵਿਕਾਸ,
- ਨਾੜੀ ਮਾਸਪੇਸ਼ੀ ਦੇ ਵਿਕਾਸ ।
ਪ੍ਰਸ਼ਨ 2.
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਖੇਤਰ ਲਿਖੋ ।
ਉੱਤਰ-
ਵਿਦਿਆਰਥੀਆਂ ਦਾ ਸਰਵਪੱਖੀ ਵਿਕਾਸ ਜਿਵੇਂ ਸਰੀਰਕ, ਮਾਨਸਿਕ, ਸਮਾਜਿਕ ਤੇ ਨੈਤਿਕ ਵਿਕਾਸ ਕਰਨ ਦੀ ਅਣਗਿਣਤ ਸਰੀਰਕ ਕਿਰਿਆ ਦੁਆਰਾ ਕੋਸ਼ਿਸ਼ ਕੀਤੀ ਜਾਂਦੀ ਹੈ ।
ਪ੍ਰਸ਼ਨ 3.
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਕੋਈ ਤਿੰਨ ਮਹੱਤਵ ਲਿਖੋ ।
ਉੱਤਰ-
- ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਪਾਠ-ਕ੍ਰਮ ।
- ਸਰੀਰਕ ਸਿੱਖਿਆ ਵਿਚ ਫ਼ਿਰਕੂਪੁਣੇ ਲਈ ਕੋਈ ਸਥਾਨ ਨਹੀਂ ।
- ਦੇਸ਼ ਭਗਤੀ, ਅਨੁਸ਼ਾਸਨ ਅਤੇ ਸਹਿਣਸ਼ੀਲਤਾ ।
ਛੋਟੇ ਉੱਤਰਾਂ ਵਾਲੇ ਪ੍ਰਸ਼ਨ (Short Answer Type Questions)
ਪ੍ਰਸ਼ਨ 1.
ਜੇ. ਐੱਫ਼ ਵਿਲੀਅਮਜ਼ ਦੇ ਅਨੁਸਾਰ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਟੀਚਾ ਕੀ ਹੈ ?
ਉੱਤਰ-
ਜੇ.ਐੱਫ਼ ਵਿਲੀਅਮਜ਼ ਦੇ ਵਿਚਾਰ (Views of J.F. Williams)-
‘‘ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਟੀਚਾ ਇਕ ਕੁਸ਼ਲ ਅਗਵਾਈ ਉੱਚਿਤ ਸਹੂਲਤਾਂ ਅਤੇ ਕਾਫ਼ੀ ਸਮਾਂ ਦੁਆਉਣਾ ਹੈ ਜਿਸ ਨਾਲ ਵਿਅਕਤੀ ਜਾਂ ਸੰਗਠਨਾਂ ਨੂੰ ਇਸ ਤਰ੍ਹਾਂ ਦੀਆਂ ਸਥਿਤੀਆਂ ਵਿਚ ਭਾਗ ਲੈਣ ਦਾ ਅਵਸਰ ਮਿਲ ਸਕੇ ਜੋ ਸਰੀਰਕ ਰੂਪ ਨਾਲ ਆਨੰਦਦਾਇਕ ਮਾਨਸਿਕ ਰੂਪ ਨਾਲ ਪ੍ਰੇਰਕ ਤੋਂ ਸੰਤੋਖਜਨਕ ਅਤੇ ਸਮਾਜਿਕ ਰੂਪ ਨਾਲ ਤੰਦਰੁਸਤ ਹੋਣ ।” .
![]()
ਪ੍ਰਸ਼ਨ 2.
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਸਲਾਹਕਾਰ ਬੋਰਡ ਦੇ ਅਨੁਸਾਰ ਸਰੀਰ ਸਿੱਖਿਆ ਦੀ ਪਰਿਭਾਸ਼ਾ ਲਿਖੋ ।
ਉੱਤਰ-
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਸਲਾਹਕਾਰ ਬੋਰਡ ਦੇ ਵਿਚਾਰ (Views of central Advisory Board of physical education) – ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਉਦੇਸ਼ ਹਰੇਕ ਬੱਚੇ ਨੂੰ ਸਰੀਰਕ, ਮਾਨਸਿਕ ਅਤੇ ਭਾਵਾਤਮਕ ਤੌਰ ‘ਤੇ ਯੋਗ ਬਣਾਉਣਾ ਹੈ ਅਤੇ ਉਸ ਵਿਚ ਕਈ ਤਰ੍ਹਾਂ ਦੇ ਨਿੱਜੀ ਅਤੇ ਸਮਾਜਿਕ ਗੁਣ ਵਿਕਸਿਤ ਕਰਨਾ ਹੈ ਜਿਸ ਨਾਲ ਉਹ ਸਮਾਜ ਦੇ ਦੂਜੇ ਮੈਂਬਰਾਂ ਦੇ ਨਾਲ ਪ੍ਰਸੰਨਤਾ ਪੂਰਵਕ ਰਹਿ ਸਕੇ ਅਤੇ ਚੰਗਾ ਨਾਗਰਿਕ ਬਣ ਸਕੇ ।”
ਵੱਡੇ ਉੱਤਰ ਵਾਲਾ ਪ੍ਰਸ਼ਨ (Long Answer Type Question)
ਪ੍ਰਸ਼ਨ 1.
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਖੇਤਰ ਬਾਰੇ ਲਿਖੋ ।
ਉੱਤਰ-
ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਖੇਤਰ
(Scope of Physical Education)
ਅੱਜ ਦੇ ਯੁੱਗ ਵਿਚ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਖੇਤਰ ਵਿਚ ਕੇਵਲ ਸਰੀਰ ਦੀ ਰਚਨਾ, ਕਿਰਿਆਵਾਂ, ਜਿਮਨਾਸਟਿਕ, ਡਰਿਲ ਅਤੇ ਮਾਰਚਿੰਗ ਹੀ ਨਹੀਂ ਆਉਂਦੇ, ਬਲਕਿ ਵਿਦਿਆਰਥੀਆਂ ਦੇ ਸਰਵ-ਪੱਖੀ ਵਿਕਾਸ ਜਿਵੇਂ ਮਾਨਸਿਕ, ਸਰੀਰਕ, ਸਮਾਜਿਕ ਅਤੇ ਨੈਤਿਕ ਵਿਕਾਸ ਕਰਨ ਦੀ ਅਣਗਿਣਤ ਕਿਰਿਆਵਾਂ ਦੁਆਰਾ ਕੋਸ਼ਿਸ਼ ਕੀਤੀ ਜਾਂਦੀ ਹੈ । ਜਿਸ ਤੋਂ ਪਤਾ ਚਲਦਾ ਹੈ ਕਿ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦਾ ਖੇਤਰ ਬਹੁਤ ਵਿਸ਼ਾਲ ਹੈ । ਜਿਨ੍ਹਾਂ ਵਿਚ ਹੇਠ ਲਿਖੀਆਂ ਪ੍ਰਤੀਕਿਰਿਆਵਾਂ ਅਤੇ ਕਾਰਜ-ਕੁਮ ਆਉਂਦੇ ਹਨ-
- ਉਪਚਾਰਾਤਮਕ ਕਸਰਤਾਂ (Corrective Exercises) – ਇਨ੍ਹਾਂ ਕਸਰਤਾਂ ਨਾਲ ਵਿਦਿਆਰਥੀ ਦੇ ਸਰੀਰਕ ਅੰਗਾਂ ਦੀ ਕਮਜ਼ੋਰੀ ਅਤੇ ਕਰੂਪਤਾ ਦਾ ਉਪਚਾਰ ਕੀਤਾ ਜਾਂਦਾ ਹੈ । ਕਈ ਵਾਰੀ ਇਹ ਨੁਕਸ ਮਾਸ ਪੇਸ਼ੀਆਂ ਦੇ ਕਮਜ਼ੋਰ ਹੋਣ ਨਾਲ ਹੋ ਜਾਂਦੇ ਹਨ । ਇਨ੍ਹਾਂ ਨੂੰ ਦੂਰ ਕਰਨ ਲਈ ਹਲਕੀਆਂ ਕਸਰਤਾਂ, ਭਾਰ ਉਠਾਉਣਾ ਅਤੇ ਯੋਗ ਆਦਿ ਦੀ ਸਹਾਇਤਾ ਲਈ ਜਾਂਦੀ ਹੈ ।
- ਖੇਡ-ਕੁੱਦ ਅਤੇ ਤੈਰਾਕੀ (Games and Sports and Swimming) – ਇਸ ਵਿਚ ਅਥਲੈਟਿਕ, ਟੇਬਲ ਟੈਨਿਸ, | ਹਾਕੀ, ਫੁੱਟਬਾਲ, ਬਾਸਕਟਬਾਲ, ਤੈਰਾਕੀ, ਬੇੜੀ ਚਲਾਉਣਾ ਆਦਿ ਖੇਡਾਂ ਆਉਂਦੀਆਂ ਹਨ ।
- ਆਤਮ ਰੱਖਿਅਕ ਕਿਰਿਆ (Self-defence activities) – ਇਸ ਵਿਚ ਵੰਡ ਕੱਢਣਾ, ਕੁਸ਼ਤੀਆਂ, ਮੁੱਕੇਬਾਜ਼ੀ, | ਸੋਟੀ ਚਲਾਉਣਾ, ਗਤਕਾ ਅਤੇ ਪੁਲ ਅੱਪ ਆਦਿ ਕਰਵਾਈਆਂ ਜਾਂਦੀਆਂ ਹਨ ।
- ਬੁਨਿਆਦੀ ਜਿਮਨਾਸਟਿਕ (Fundamental Gymnastics) – ਇਸ ਖੇਤਰ ਵਿਚ ਸਰੀਰ ਦਾ ਸੰਤੁਲਨ ਠੀਕ ਰੱਖਿਆ ਜਾਂਦਾ ਹੈ । ਸੰਤੁਲਨ ਲਈ ਚਲਣਾ, ਫਿਰਨਾ, ਦੌੜਨਾ, ਚੜ੍ਹਨਾ, ਉਤਰਨਾ ਆਦਿ ਕਿਰਿਆਵਾਂ ਸ਼ਾਮਿਲ ਹਨ ।
- ਤਾਲ ਅਤੇ ਨਾਚ (Rythmics) – ਇਸ ਵਿਚ ਲੇਜੀਅਮ, ਟਿਪਰੀ ਅਤੇ ਨਾਚ ਜਿਵੇਂ ਲੋਕ ਨਾਚ, ਮਨੋਰੰਜਨ ਅਤੇ ਸੰਗੀਤ ਉੱਤੇ ਜਿਮਨਾਸਟਿਕ ਸ਼ਾਮਿਲ ਹਨ ।
- ਮਨੋਰੰਜਕ ਕਿਰਿਆਵਾਂ (Recreation – ਇਸ ਵਿਚ ਕੈਂਪ ਲਾਉਣਾ, ਦੂਰ ਤਕ ਸੈਰ ਕਰਨਾ, ਮੱਛੀਆਂ ਫੜਨਾ ਅਤੇ ਕੁਦਰਤ ਦੀ ਜਾਣਕਾਰੀ ਸ਼ਾਮਿਲ ਹੈ ।
- ਯੋਗ ਕਿਰਿਆਵਾਂ (Yoga) – ਇਸ ਵਿਚ ਅਲੱਗ-ਅਲੱਗ ਆਸਨ, ਪ੍ਰਾਣਾਯਾਮ ਅਤੇ ਹੋਰ ਯੋਗਿਕ ਕਿਰਿਆਵਾਂ ਸ਼ਾਮਿਲ ਹਨ ।
ਸਮੁੱਚੇ ਤੌਰ ‘ਤੇ ਅਸੀਂ ਕਹਿ ਸਕਦੇ ਹਾਂ ਕਿ ਉਪਰੋਕਤ ਕਿਰਿਆਵਾਂ ਸਰੀਰਕ ਸਿੱਖਿਆ ਦੇ ਖੇਤਰ ਵਿਚ ਇਹੋ ਜਿਹੀਆਂ ਕਿਰਿਆਵਾਂ ਹਨ ਜਿਨ੍ਹਾਂ ਨੂੰ ਕਰਨ ਨਾਲ ਮਨੁੱਖ ਦਾ ਬਹੁ-ਪੱਖੀ ਵਿਕਾਸ ਆਸਾਨੀ ਨਾਲ ਕੀਤਾ ਜਾ ਸਕਦਾ ਹੈ ।