Punjab State Board PSEB 12th Class Chemistry Important Questions Chapter 5 Surface Chemistry Important Questions and Answers.
PSEB 12th Class Chemistry Important Questions Chapter 5 Surface Chemistry
Very Short Answer Type Questions
Question 1.
Define desorption.
Answer:
The process of removal of an adsorbed substance from a surface on which it is adsorbed is called desorption.
Question 2.
What is the effect of temperature on chemisorption?
Answer:
Chemisorption initially increases then decreases with rise in temperature. The initial increase is due to the fact that heat supplied acts as activation energy. The decrease afterwards is due to the exothermic nature of adsorption equilibrium.
Question 3.
What is the role of diffusion in heterogeneous catalysis?
Answer:
The gaseous molecules diffuses on to the surface of the solid catalyst and get adsorbed. After the required chemical changes, the products diffuse away from the surface of the catalyst leaving the surface free for more reactant molecules to get adsorbed and undergo reaction.
![]()
Question 4.
What is the type of charge on Agl colloidal sol formed when AgNO3 solution is added to KI solution?
Answer:
Negatively charged sol, Agl/I– is formed when AgNO3 solution is added to KI solution.
Question 5.
What causes Brownian movement in a colloidal solution?
Answer:
Unbalanced bombardment of the particles of dispersed phase by molecules of dispersion medium causes Brownian motion. This stabilises the sol.
Question 6.
Based on the type of dispersed phase, what type of colloid is micelles?
Answer:
Associated colloids
![]()
Question 7.
Name the temperature above which the formation of micelles takes place.
Answer:
Kraft temperature.
Question 8.
How do emulsifying agents stabilise the emulsion?
Answer:
The emulsifying agent forms an interfacial layer between suspended particles and the dispersion medium thereby stabilising the emulsion.
Question 9.
Write the dispersed phase and dispersion medium of butter.
Answer:
Dispersed phase — Liquid
Dispersion medium — Solid.
![]()
Question 10.
Write the main reason for the stability of colloidal sols.
Answer:
All the particles of colloidal sol carry the same charge so they keep on repelling each other and do not aggregate together to form bigger particles.
Question 11.
How is Brownian movement responsible for the stability of sols?
Answer:
The Brownian movement has a stirring effect, which does not allow the particles to settle down.
Short Answer Type Questions
Question 1.
Differentiate among a homogeneous solution, a suspension and a colloidal solution, giving a suitable example of each.
Answer:
| Property |
Homogeneous solution |
Colloidal solution |
Suspension |
| (i) Particle size | Less than 1 nm | Between 1 nm to 1000 nm | More than 1000 nm |
| (ii) Separation by | |||
| ordinary filtration | Not possible | Not possible | Not possible |
| ultra filtration | Not possible | Possible | Possible |
| (iii) Settling of particles | Do not settle | Settle only on coagulation | Settle under gravity |
| (iv) Appearance | Transparent | Opaque | Translucent |
| (v) Example | Glucose dissolved in water | Smoke, milk, gold sol | Sand in water |
![]()
Question 2.
Classify colloids where the dispersion medium is water. State their characteristics and write an example of each of these classes.
Answer:
These are of two types
(i) Hydrophilic
Stability: More stable as the stability is due to charge and water envelope surrounding the sol particles.
Nature: Reversible
Examples: Starch, gum etc.
(ii) Hydrophobic
Stability: Less stable as the stability is due to charge only.
Nature: Irreversible
Examples: Metal hydroxide like Fe(OH)3 and metal sulphide like As2S3.
Question 3.
Explain the cleansing action of soap. Why do soaps not work in hard water?
Answer:
The cleansing action of soap such as sodium stearate is due to the fact that soap molecules form micelle around the oil droplet in such a way that hydrophobic part of the stearate ions is in the oil droplet and hydrophilic part projects out of the grease droplet like the bristles. Since the polar groups can interact with water, the oil droplet surrounded by stearate ions is now pulled in water and removed from the dirty surface. Thus, soap helps in emulsification and washing away of oils and fats.
Hard water contains calcium and magnesium salts. In hard water, soap gets precipitated as calcium and magnesium soap which being insoluble stick to the clothes as gummy mass. Therefore, soaps do not work in hard water.
Question 4.
Adsorption of a gas on the surface of solid is generally accompanied by a decrease in entropy still it is a spontaneous process. Why?
Answer:
According to the equation
△G = △H – T△S
For a process to be spontaneous, △G should be negative. Even though △S is negative here, △G is negative because reaction is highly exothermic, i.e., △H is negative.
![]()
Question 5.
Define the following terms:
(i) Brownian movement,
(ii) Peptization.
Answer:
(i) Brownian movement : The motion of the colloidal particles in a zig zag path due to unbalanced bombardment by the particles of dispersion medium is called Brownian movement.
(ii) Peptization : The process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of a small amount of suitable electrolyte is called peptization. During peptization, the precipitate absorbs one of the ions of the electrolyte on its surface. This causes development of positive or negative charge on precipitates, which ultimately break up into particles of colloidal dimension.
Question 6.
(i) Write the expression for Freundlich’s equation to describe the behaviour of adsorption from solution.
(ii) What causes charge on sol particles?
(iii) Name the promoter used in the haber’s process for the manufacture of ammonia.
Answer:
(i) \(\frac{x}{m}\) = KC\(\frac{1}{n}\)
(ii) The charge on the sol particles is due to :
- electron capture by sol particles during electro dispersion.
- preferential anolsorption of ions from solution.
- formulation of electrical double layer.
(iii) Molybdenum acts in a promoter for iron.
![]()
Long Answer Type Questions
Question 1.
Consider the adsorption isotherms given alongside and interpret the variation in the extent of adsorption (xlm) when
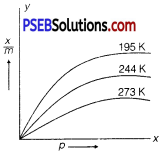
(a) (i) temperature increases at constant pressure.
(ii) pressure increases at constant temperature.
(b) Name the catalyst and the promoter used in Haber’s process for manufacture of ammonia.
Answer:
(a) (i) At constant pressure, extent of adsorption \(\left(\frac{x}{m}\right)\) decreases with increase in temperature as adsorption is an exothermic process.
(ii) At constant temperature, first adsorption \(\left(\frac{x}{m}\right)\) increases with increase in pressure up to a particular pressure and then it
At low pressure, \(\frac{x}{m}\) = kp m
At intermediate range of pressure, \(\frac{x}{m}\) = kp1/n (n > 1)
At high pressure, \(\frac{x}{m}\) = k (independent of pressure)
(b) Finely divided iron is used as a catalyst and molybdenum is used as promoter.
![]()
Question 2.
Explain the following observations:
(i) Sun looks red at the time of setting.
(ii) Cottrell’s smoke precipitator is fitted at the mouth of the chimney used in factories.
(iii) Physical adsorption is multilayered while chemical adsorption is monolayered.
Answer:
(i) At the time of setting, the sun is at horizon. The light emitted by the sun has to travel a relatively longer distance through the atmosphere. As a result, blue part of light is scattered away by the particulate in the atmosphere causing red part to be visible.
(ii) Cottrell’s smoke precipitator, neutralises the charge on unburnt carbon particles, coming out of chimney and they get precipitated and settle down at the floor of the chamber.
(iii) Physical adsorption involves van der Waals’ forces, so any number of layers may be formed one over the other on the surface of the adsorbent. Chemical adsorption takes place as a result of the reaction between adsorbent and adsorbate. When the surface of adsorbent is covered with one layer, no further reaction can take place.