Punjab State Board PSEB 11th Class Chemistry Book Solutions Chapter 9 Hydrogen Textbook Exercise Questions and Answers.
PSEB Solutions for Class 11 Chemistry Chapter 9 Hydrogen
PSEB 11th Class Chemistry Guide Hydrogen InText Questions and Answers
Question 1.
Justify the position of hydrogen in the periodic table on the basis of its electronic configuration.
Answer:
Hydrogen is the first element in the periodic table. It has the electronic configuration 1s1. It is similar to alkali metal (ns1) of group I. It shows resemblance with alkali metals of group I of the periodic table. So it can be placed above the alkali metals in group I of the periodic table.
On the other hand, the electronic configuration of hydrogen shows that it is short of one electron to the nearest noble gas configuration (He) having the electronic configuration 1s2. Like halogens it forms covalent bonds (H2, Cl2, Br2, etc.) as well as ionic bonds (e.g. Na+ H–). It forms H+ ion by giving one electron and hydride ion (H–) by gaining one electron. On the basis of its electronic configuration (1s2) hydrogen is placed with other ns1 elements namely alkali metals in the group I as well as in group 17 of the periodic table. Thus, the position of hydrogen in the periodic table is anomalous.
Hydrogen with so many unique characteristics is, therefore best placed separately in the periodic table of elements.
![]()
Question 2.
Write the names of isotopes of hydrogen. What is the mass ratio of these isotopes?
Answer:
Hydrogen has following three isotopes :
1. Protium,\({ }_{1}^{1} \mathrm{H}\),
2. Deuterium, \({ }_{1}^{2} \mathrm{H}\) or D, and
3. Tritium, \({ }_{1}^{3} \mathrm{H}\) or T
The mass ratio of protium, deuterium and tritium is 1.008 : 2.014 : 3.016 or 1:2:3.
Question 3.
Why does hydrogen occur in a diatomic form rather than in a monoatomic form under normal conditions?
Answer:
The ionization enthalpy of hydrogen atom is very high (1312 kJ mol-1). Hence, it is very hard to remove its electron. As a result, its tendency to exist in the monoatomic form is rather low. Instead, hydrogen forms a covalent bond with another hydrogen atom and exists as a diatomic (H2) molecule.
Question 4.
How can the production of dihydrogen, obtained from ‘coal gasification’, be increased?
Answer:
Dihydrogen, produced by coal gasification method as :
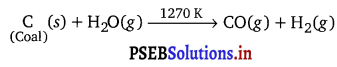
The yield of dihydrogen (obtained from coal gasification) can be increased by reacting carbon monoxide (formed during the reaction) with steam in the presence of iron chromate as a catalyst.
![]()
This reaction is called the water-gas shift reaction. Carbon dioxide is removed by scrubbing it with a solution of sodium arsenite.
Question 5.
Describe the bulk preparation of dihydrogen by electrolytic method. What is the role of an electrolyte in this process?
Answer:
Electrolysis of acidified water using platinum electrodes gives dihydrogen.
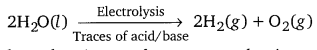
The role of an electrolyte is to make water conducting.
Question 6.
Complete the following reactions :
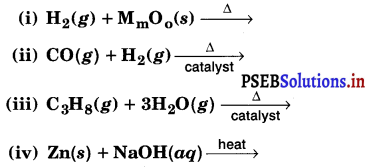
Answer:
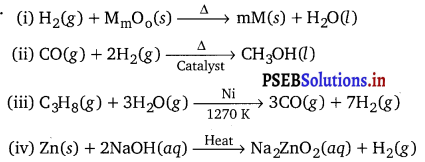
![]()
Question 7.
Discuss the consequences of high enthalpy of H-H bond in terms of chemical reactivity of dihydrogen.
Answer:
The ionization enthalpy of H-H bond is very high (1312 kJ mol-1). This indicates that dihydrogen has a low tendency to form H+ ions. Its ionization enthalpy value is comparable to that of halogens. Hence, it forms diatomic molecules (H2), hydrides with elements, and a large number of covalent bonds.
Since ionization enthalpy is very high, dihydrogen does not possess metallic characteristics (lustre, ductility, etc.) like metals.
Question 8.
What do you understand by (i) electron-deficient,
(ii) electron-precise, and (iii) electron-rich compounds of hydrogen? Provide justification with suitable examples.
Answer:
(i) An electron-deficient hydride : It has very few electrons, less than that required for representing its conventional Lewis structure e.g., diborane (B2H6). In B2H6, there are six bonds in all, out of which only four bonds are regular i.e., two electrons are shared by two atoms.
The remaining two bonds are three centered-two electron bonds i.e., two electrons are shared by three atoms. Hence, its conventional Lewis structure cannot be drawn.
(ii) An electron-precise hydride : It has sufficient number of electrons to be represented by its conventional Lewis structure e.g., CH4. The Lewis structure can be written as :
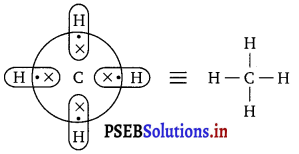
Four regular bonds are formed where two electrons are shared by two atoms.
(iii) An electron-rich hybride : It contains excess electrons as lone pair e.g., NH3
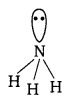
There are three regular bonds in all with a lone pair of electrons on the nitrogen atom.
Question 9.
What characteristics do you expect from an electron-deficient hydride with respect to its structure and chemical reactions?
Answer:
These hydrides do not have sufficient number of electrons to form normal covalent bonds, e.g., B in BF3 has 6 electrons in its valence shell. These hydrides are trigonal planar in shape.
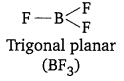
These hydrides act as Lewis acids, i.e., electron pair acceptor e.g.,
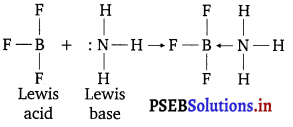
To make up the deficiency of electrons, these hydrides exist in polymeric forms e.g., B2H6, B4H10, etc. Electron deficient hydrides are very reactive. These reacts readily with metals and non-metals and their compounds, e.g.,
B2H6 + 3O2(g) → B2O3(s) + 3H2O(g)
Question 10.
Do you expect the carbon hydrides of the type (CraH2jt + 2) to act as ‘Lewis’ acid or base? Justify your answer.
Answer:
Carbon hydrides of the type CnH2n+2 are CH4, C2H6 etc. in which number of electrons present are just sufficient to write down their conventional Lewis structures.
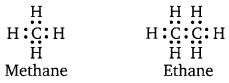
C has neither extra electrons nor less electrons. Such compounds of the formula CnH2n+2 are called ELECTRON-PRECISE compounds. They will act neither as Lewis acids nor as Lewis bases.
Question 11.
What do you understand by the term “non-stoichiometric hydrides”? Do you expect this type of the hydrides to be formed by alkali metals? Justify your answer.
Answer:
Non-stoichiometric hydrides are hydrogen-deficient compounds formed by the reaction of dihydrogen with d-block and f-block elements. These hydrides do not follow the law of constant composition.
For example :
LaH287, YbH2.55, TiH1.5 – 1.8 etc.
Alkali metals form stoichiometric hydrides. These hydrides are ionic in nature. Hydride ions have comparable sizes (208 pm) with alkali metal ions. Hence, strong binding forces exist between the constituting metal and hydride ion. As a result, stoichiometric hydrides are formed. Alkali metals will not form non-stoichiometric hydrides.
Question 12.
How do you expect the metallic hydrides to be useful for hydrogen storage? Explain.
Answer:
Metallic hydrides are hydrogen deficient, i.e., they do not hold the law of constant composition. It has been established that in the hydrides of Ni, Pd, Ce and Ac hydrogen occupies the interstitial position in lattices allowing further absorption of hydrogen on these metals. Metals like Pd, Pt, etc. have the capacity to accommodate a large volume of hydrogen. Therefore, they are used for the storage of hydrogen and serve as a source of energy.
![]()
Question 13.
How does the atomic hydrogen or oxy-hydrogen torch function for cutting and welding purposes? Explain.
Answer:
Atomic hydrogen atoms are produced by the dissociation of dihydrogen with the help of an electric arc. This releases a huge amount of energy (435.88 kJ mol-1). This energy can be used to generate a temperature of 4000 K, which is ideal for welding and cutting metals. Hence, atomic k hydrogen or oxy-hydrogen torches are used for these purposes. For this reason, atomic hydrogen is allowed to recombine on the surface to be welded to generate the desired temperature.
Question 14.
Among NH3, H2O and HF, which would you expect to have highest magnitude of hydrogen bonding and why?
Answer:
The extent of hydrogen bonding depends upon electronegativity and the number of hydrogen atoms available for bonding. Among nitrogen, fluorine, and oxygen, the increasing order of their electronegativities are N < O < F. Hence, the order of the extent of hydrogen bonding is HF > H2O > NH3.
Question 15.
Saline hydrides are known to react with water violently producing fire. Can CO2, a well known fire extinguisher, be used in this case? Explain.
Answer:
Saline hydrides (i.e., NaH, LiH, etc.) react with water to form a base and hydrogen gas. The chemical equation used to represent the reaction can be written as:
NaH(s) + H2O(7) → NaOH(aq) + H2(g)
This reaction is violent and produces fire.
This type of fire cannot be extinguished by CO2 because it gets reduced by the hot metal hydride to form sodium format.
NaH + CO2 → HCOONa
Question 16.
Arrange the following
(i) CaH2, BeH2 and TiH2 in order of increasing electrical conductance.
(ii) LiH, NaH and CsH in order of increasing ionic character.
(iii) H-H, D-D and F-F in order of increasing bond dissociation enthalpy.
(iv) NaH, MgH2 and H2O in order of increasing reducing property.
Answer:
(i) BeH2 < CaH2 < TiH2
(ii) LiH < NaH < CsH
(iii) F—F < H—H < D—D
(iv) H2O < MgH2 < NaH
Question 17.
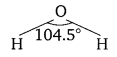
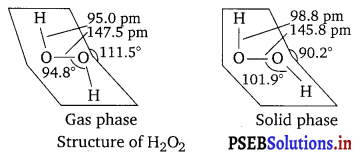
Compare the structures of H2O and H2O2.
Answer:
In gaseous phase, water molecule has a bent form with a bond angle of 104.5°. The O—H bond length is 95.7 pm. The structure can be shown as:
Hydrogen peroxide has a non-planar structure both in gas and solid phase. The dihedral angle in gas and solid phase is 111.5° and 90.2° respectively.
Question 18.
What do you understand by the term ‘auto-protolysis’ of water? What is its significance?
Answer:
Auto-protolysis (self-ionization) of water is a chemical reaction in which two water molecules react to produce a hydroxide ion (OH–) and a hydronium ion (H3O+).
The reaction involved can be represented as :
![]()
Auto-protolysis of water indicates its amphoteric nature i.e., its ability to act as an acid as well as a base. The acid-base reaction can be written as :
![]()
Question 19.
Consider the reaction of water with F2 and suggest, in terms of oxidation and reduction, which species are oxidized/reduced.
Answer:
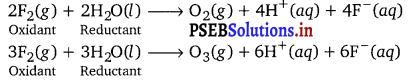
In these reactions, water acts as a reducing agent and hence itself gets oxidised to either oxygen or ozone. Fluorine acts as an oxidising agent and hence itself reduced to F– ion.
![]()
Question 20.
Complete the following chemical reactions.
(i) PbS(s) + H2O2(ciq) →
(ii) MnO–4(aq) + H2O2(aq) →
(iii) CaO(s) + H2O(g) →
(iv) AlCl3(g) + H2O(Z) →
(v) Ca3N2(s) + H2O(l) →
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
Answer:
(i) PbS(s) + 4H2O2(aq) → PbSO4(s) + 4H2O(l)
H2O2 is acting as an oxidizing agent in the reaction. Hence, it is a redox reaction.
(ii) 2MnO4– (aq) + 5H2O2(Z) + 6H+(aq) → 2Mn2+(aq) + 8H2O(Z) + 5O2(g)
H2O2 is acting as a reducing agent in the acidic medium, thereby oxidizing MnO–4(aq). Hence, the given reaction is a redox reaction.
(iii) CaO(s) + H2O(g) → Ca(OH)2(aq)
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction is hydrolysis reaction.
(iv) AlCl3(g) + 6H2O(Z) → [Al(OH2)6]3+ (aq) + 3Cl–(aq)
It is a hydration reaction, because A1C13 is hydrated to [Al(OH2)6]3+.
(v) Ca3N2(s) + 6H2O(Z) → 3Ca(OH)2(aq) + 2NH3(aq)
The reactions in which a compounds reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of Ca3N2.
Question 21.
Describe the structure of the common form of ice.
Answer:
Ice is the crystalline form of water. It takes a hexagonal form if crystallized at atmospheric pressure, but condenses to cubic form if the temperature is very low.
The three-dimensional structure of ice is represented as :
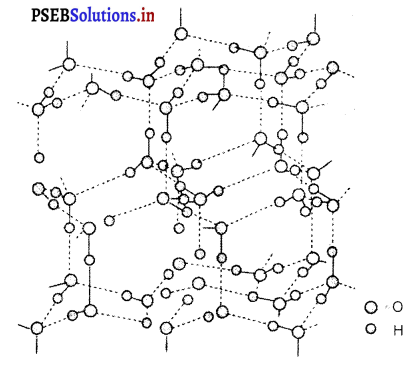
The structure is highly ordered and has hydrogen bonding. Each oxygen atom is surrounded tetrahedrally by four other oxygen atoms at a distance of 276 pm. The structure also contains wide holes that can hold molecules of appropriate sizes interstitially.
Question 22.
What causes the temporary and permanent hardness of water?
Answer:
Temporary hardness of water is due to the presence of soluble salts of magnesium and calcium in the form of hydrogen carbonates (MHCO3, where M = Mg, Ca) in water.
Permanent hardness of water is due to the presence of soluble salts of calcium and magnesium in the form of chlorides in water.
Question 23.
Discuss the principle and method of softening of hard water by synthetic ion-exchange resins.
Answer:
The process of treating permanent hardness of water using synthetic resins is based on the exchange of cations (e.g., Na+, Ca2+, Mg2+ etc.) and anions (e.g.,Cl– , \(\mathrm{SO}_{4}^{2-}\), \(\mathrm{HCO}_{3}^{-}\) etc.) present in water by H+ and OH– ions respectively.
Synthetic resins are of two types :
1. Cation exchange resins
2. Anion exchange resins
Cation exchange resins are large organic molecules that contain the -SO3H group. The resin is firstly changed to RNa (from ROS3H) by treating it with NaCl. This resin then exchanges Na+ ions with Ca2+ and Mg2+ ions, thereby making the water soft.
2RNa + M2+ (aq) → R2M(s) + 2Na+ (aq)
There are cation exchange resins in H+ form. The resins exchange H+ ions for Na+, Ca2+ and Mg2+ ions.
2RH + M2+ (aq) ⇌ MR2(s) + 2H+(aq)
Anion exchange resins exchange OH- ions for anions like Cl–, \(\) and \(\) present in water.
![]()
During the complete process, water first passes through the cation exchange process. The water obtained after this process is free from mineral cations and is acidic in nature.
This acidic water is then passed through the anion exchange process where OH– ions neutralize the H+ ions and de-ionize the water obtained.
![]()
Question 24.
Write chemical reactions to show the amphoteric nature of water.
Answer:
Water is amphoteric in character. It behaves both as an acid as well as a base. With acids stronger than itself, it behaves as a base and with bases stronger than itself, it acts as an acid.
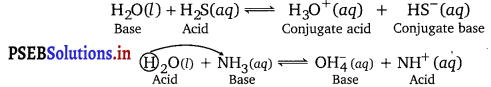
Question 25.
Write chemical reactions to justify that hydrogen peroxide can function as an oxidizing as well as reducing agent.
Answer:
Hydrogen peroxide, H2O2 acts as an oxidizing as well as a reducing agent in both acidic and alkaline media. Reactions involving oxidizing actions are :
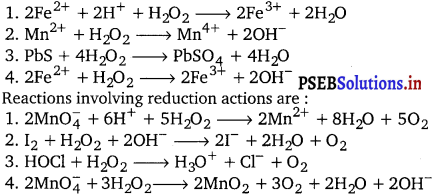
Question 26.
What is meant by ‘demineralised’ water and how can it be obtained?
Answer:
Water which does not contain cations and anions is called ‘demineralised’ water. It is soft water. Demineralised water is obtained the same way as soft water is obtained from hard water. Demineralised or deionised water is obtained by passing hard water first through a cation exchange resin (RCOOH or RSO3H) which removes Ca2+ and Mg2+ ions from hard water by exchanging them with H+ ions and then passing through an anion exchange resin ![]() which removes Cl– and \(\mathrm{SO}_{4}^{2-}\) ions present in hard water by exchanging them with OH– ions.
which removes Cl– and \(\mathrm{SO}_{4}^{2-}\) ions present in hard water by exchanging them with OH– ions.
Question 27.
Is demineralised or distilled water useful for drinking purposes? If not, how can it be made useful?
Answer:
Water is an important part of life. It contains several dissolved nutrients that are required by human beings, plants and animals for survival.
Demineralised water is free from all soluble minerals. Hence, it is not fit for drinking.
It can be made useful only after the addition of desired minerals in specific amounts, which are important for growth.
Question 28.
Describe the usefulness of water in biosphere and biological systems.
Answer:
Water is essential for all forms of life. It constitutes around 65% of the human body and 95% of plants. Water plays an important role in the biosphere owing to its high specific heat, thermal conductivity, surface tension, dipole moment and dielectric constant.
The high heat of vaporization and heat of capacity of water helps in moderating the climate and body temperature of all living beings.
It acts as a carrier of various nutrients required by plants and animals for various metabolic reactions.
Water is also required for photosynthesis in plants which releases O2 into the atmosphere.
![]()
Question 29.
What properties of water make it useful as a solvent? What types of compound can it (i) dissolve, and (ii) hydrolyse?
Answer:
Water because of its high dielectric constant (78.39) has the ability to dissolve most of the inorganic (ionic) compounds and is, therefore, regarded as a universal solvent. Whereas the solubility of ionic compounds takes place due to ion-dipole interactions (i.e., solvation of ions) the solubility of covalent compounds such as alcohols, amines, urea, glucose, sugar etc. takes place due to tendency of these molecules to form hydrogen bonds with water.
(i) It can dissolve both ionic compounds as well as covalent compounds which can form hydrogen bonds with water.
Ionic compounds whose lattice energy is lower than hydration energy get dissolved in water.
(ii) Water can hydrolyse many oxides (metallic and non-metallic), hydrides, carbides, nitrides, phosphides and many other salts e.g.,
CaO(s) + H2O(l) → Ca(OH)2
SO2(g) + H2O(l) → H2SO3(aq)
CaH2(s) + 2H2O(l) → Ca(OH)2(uq) + 2H2(g)
SiCl4(l) + 4H2O(l) → SiO2 -2H2O(s) + 4HCl(aq)
Al4C3 + 12H2O(l) → 4Al(OH)3 + 3CH4
Ca3P2(s) + 6H2O → 3Ca(OH)2 + 2PH3
Question 30.
Knowing the properties of H20 and DaO, do you think that D20 can be used for drinking purposes?
Answer:
Heavy water (D2O) acts as a moderator, i.e., it slows the rate of a reaction. Due to this property of D2O, it cannot be used for drinking purposes because it will slow down anabolic and catabolic reactions takes place in the body and lead to a casualty.
Question 31.
What is the difference between the terms ‘hydrolysis’ and ‘hydration’?
Answer:
Hydrolysis is defined as a chemical reaction in which hydrogen and hydroxide ions (H+ and OH- ions) of water molecule react with a compound to form products. For example :
NaH + H2O → NaOH + H2
Hydration is defined as the addition of one or more water molecules to ions or molecules to form hydrated compounds. For example :
CUSO4 + 5H2O → CUSO4 . 5H2O
![]()
Question 32.
How can saline hydrides remove traces of water from organic compounds? ’
Answer:
Saline hydrides are ionic in nature. They react with water to form a metal hydroxide along with the liberation of hydrogen gas. The reaction of saline hydrides with water can be represented as :
AH(s) + H20(Z) → AOH(aq) + H2(g)
(where, A= Na, Ca, )
When added to an organic solvent, they react with water present in it. Hydrogen escapes into the atmosphere leaving behind the metallic hydroxide.Then, the dry organic solvent distills over.
Question 33.
What do you expect the nature of hydrides is, if formed by elements of atomic number 15, 19, 23 and 44 with dihydrogen? Compare their behaviour towards water,
Answer:
The elements of atomic number 15, 19, 23 and 44 are nitrogen, potassium, vanadium and ruthenium respectively.
1. Hydride of nitrogen
Hydride of nitrogen (NH3) is a covalent molecule. It is an electron-rich hydride owing to the presence of excess electrons as a lone pair on nitrogen.

2. Hydride of potassium
Dihydrogen forms an ionic hydride with potassium owing to the high electropositive nature of potassium. It is crystalline and non-volatile in nature.
3. Hydrides of Vanadium and Ruthenium
Both vanadium and ruthenium belong to the d-block of the periodic table. The metals of d-block form metallic or non-stoichiometric hydrides. Hydrides of vanadium and ruthenium are therefore, metallic in nature having a deficiency of hydrogen.
4. Behaviour of hydrides towards water
Potassium hydride reacts violently with water as :
KH(s) + H2O(aq) → KOH(aq) + H2(g)
Ammonia (NH3) behaves as a Lewis base and reacts with water as :
H2O(Z) + NH3(aq) ⇌ OH–(aq) + \(\mathrm{NH}_{4}^{+}\)(aq)
Hydrides of vanadium and ruthenium do not react with water. Hence, the increasing order of reactivity of the hydrides is as: H < NH3 < KH.
Question 34.
Do you expect different products in solution when aluminium (III) chloride and potassium chloride treated separately with
(i) normal water (ii) acidified water, and (iii) alkaline water? Write equations wherever necessary.
Answer:
Potassium chloride (KCl) is the salt of a strong acid (HCl) and strong base (KOH). Hence, it is neutral in nature and does not undergo hydrolysis in normal water. It dissociates into ions as follows :
![]()
In acidified and alkaline water, the ions do not react and remain as such. Aluminium (III) chloride is the salt of a strong acid (HCl) and weak base [Al(OH)3]. Hence, it undergoes hydrolysis in normal water.
![]()
In acidified water, H+ ions react with Al(OH)3 forming water and giving Al3+ ions. Hence, in acidified water, AlCl3 will exist as Al3+(aq) and Cl–(aq) ions.
![]()
In alkaline water the following reaction takes place :
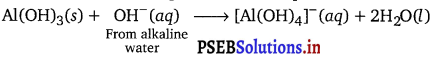
Question 35.
How does H2O2 behave as a bleaching agent?
Answer:
H2O2 acts as a bleaching agent due to the nascent oxygen.
H2O2 → H2O + O
Coloured matter + [O] → Colourless matter
It bleaches materials like silk, hair, ivory, cotton, wool, etc.
![]()
Question 36.
What do you understand by the terms :
(i) hydrogen economy (ii) hydrogenation (iii) ‘syngas’ (iv) water-gas shift reaction (v) fuel-cell?
Answer:
(i) Hydrogen economy : Hydrogen economy is a technique of using dihydrogen in an efficient way. It involves transportation and storage of dihydrogen in the form of liquid or gas.
Dihydrogen releases more energy than petrol and is more eco-friendly. Hence, it can be used in fuel cells to generate electric power. Hydrogen economy is about the transmission of this energy in the form of dihydrogen.
(ii) Hydrogenation : It refers to the addition of dihydrogen to another reactant. This process is used to reduce a compound in the presence of a suitable catalyst. For example, hydrogenation of vegetable oil using nickel as a catalyst gives edible fats such as vanaspati ghee etc.
(iii) Syngas : Syngas is a mixture of carbon monoxide and dihydrogen. Since the mixture of the two gases is used for the synthesis of methanol, it is called syngas, synthesis gas, or water gas.
Syngas is produced on the action of steam with hydrocarbons or coke at a high temperature in the presence of a catalyst.
(iv) Water gas shift reaction : The production of hydrogen by reacting carbon monoxide (CO) of syngas mixtures with steam in the presence of iron chromate as catalyst is called water-gas shift reaction.
![]()
CO2 is removed by scrubbing with sodium arsenite solution.
(v) Fuel cell: It is a device which converts the energy produced during the combustion of a fuel directly into electrical energy. One such fuel cell is hydrogen-oxygen fuel cell. It does not cause any pollution. Fuel cells generated electricity with conversion efficiency of 70-85%.