Punjab State Board PSEB 11th Class Chemistry Important Questions Chapter 5 States of Matter Important Questions and Answers.
PSEB 11th Class Chemistry Important Questions Chapter 5 States of Matter
Very Short Answer Type Questions
Question 1.
Name two intermolecular forces that exist between HF molecules in a liquid state.
Answer:
HF are polar covalent molecules. In the liquid state, there are dipole-dipole interactions and H-bonding.
Question 2.
Explain why Boyle’s law cannot be used to calculate the volume of a real gas when it is converted from its initial state to the final state by an adiabatic expansion.
Answer:
During adiabatic expansion, the temperature is lowered, and therefore, Boyle’s law cannot be applied.
Question 3.
Boyle’s law states that at constant temperature, if pressure is increased on a gas, volume decreases and vice-versa. But when we fill air in a balloon, volume as well as pressure increase. Why?
Answer:
The law is applicable only for a definite mass of the gas. As we fill air into the balloon, we are introducing more and more air into the balloon.
Thus, we are increasing the mass of air inside. Hence, the law is not applicable.
![]()
Question 4.
What will be the molar volume of nitrogen and argon at 273.15 K and 1 atm?
Answer:
Every gas has 22.4 L molar volume at 273.15 K and 1 atm pressure (STP).
Question 5.
A gas that follows Boyle’s law, Charles’ law and Avogadro’s law is called an ideal gas. Under what conditions a real gas would behave ideally?
Answer:
At low pressure and high temperature, a real gas behaves as an ideal gas.
Question 6.
Explain why temperature of a boiling liquid remains constant?
Answer:
This is because at the boiling point, the heat supplied is used up in breaking off the intermolecular forces of attraction of the liquid to change it into vapour and not for raising the temperature of the liquid.
Question 7.
Assuming C02 to be van der Waals’ gas, calculate its Boyle temperature.
Given a = 3.59 L2 atm mol-2 and b = 0.0427 L mol-1.
\(T_{b}=\frac{a}{R b}=\frac{3.59 \mathrm{~L}^{2} \mathrm{~atm} \mathrm{} \mathrm{mol}^{-2}}{\left(0.082 \mathrm{~L} \mathrm{~atm} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}\right)\left(0.0427 \mathrm{~L} \mathrm{~mol}^{-1}\right)}\) = 1025.3 K
Question 8.
Name two phenomena that can be explained on the basis of surface tension.
Answer:
Surface tension can explain
(i) capillary action, i.e., rise or fall of a liquid in capillary,
(ii) spherical shape of small liquid drops.
Question 9.
Why are the gases helium and hydrogen not liquefied at room temperature by applying very high pressure?
Answer:
Because their, critical temperature is lower than room temperature. (Gases cannot be liquefied above the critical temperature by applying even very high pressure).
Question 10.
What would have happened to the gas if the molecular collisions were not elastic?
Answer:
On every collision, there would have been loss of energy. As a result, the molecules would have slowed down and ultimately settle down in the vessel. Moreover, the pressure would have gradually reduced to zero.
Short Answer Type Questions
Question 1.
(i) What do you mean by ‘Surface Tension’ of a liquid?
(ii) Explain the factors which can affect the surface tension of a liquid.
Answer:
(i) Surface tension : It is defined as the force acting per unit length perpendicular to the line drawn on the surface. It’s unit is Nm-1.
(ii) Surface tension of a liquid depends upon the following factors :
(a) Temperature : Surface tension decreases with rise in temperature. As the temperature of the liquid increases, the average kinetic energy of the molecules increases. Thus, there is a decrease in intermolecular force of attraction which decrease the surface tension.
(b) Nature of the liquid : Greater the magnitude of
intermolecular forces of attraction in the liquid, greater will be the value of surface tension.
![]()
Question 2.
A neon-dioxygen mixture contains 70.6 g dioxygen and 167.5g neon. If pressure of the mixture of gases in the cylinder is 25 bar.
What is the partial pressure of dioxygen and neon in the mixture?
Answer:
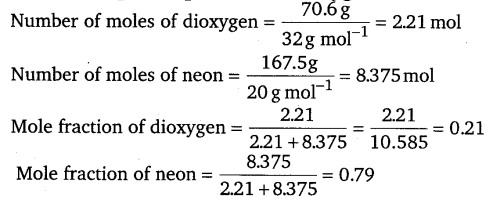
Alternatively, mole fraction of neon = 1 – 0.21 = 0.79
Partial pressure of a gas = mole fraction x total pressure
⇒ Partial pressure of oxygen = 0.21 x (25bar) = 5.25bar
Partial pressure of neon = 0.79 x (25bar) = 19.75bar
Question 3.
Give reasons for the following:
(i) The size of weather balloon becomes larger and larger as it ascends into higher altitudes.
(ii) Tyres of automobiles are inflated to lesser pressure in summer than in winter.
Answer:
(i) As we go to higher altitudes, the atmospheric pressure decreases.
Thus, the pressure outside the balloon decreases. To regain equilibrium with the external pressure, the gas inside expands to decrease its pressure, Hence, the size of the balloon increases.
(ii) In summer, due to higher temperature, the average kinetic energy of the air molecules inside the tyre increases, i.e., molecules start moving faster. Hence, the pressure on the walls of the tube increases. If pressure inside is not kept low at the time of inflation, at higher temperature, the pressure may become so high that the tyre may burst.
Question 4.
On the basis of intermolecular forces and thermal energy, explain why .
(i) a solid has rigidity but liquids do not have rigidity?
(ii) gases have high compressibility but liquids and solids have poor compressibility?
Answer:
(i) It is because in solids, the intermolecular forces are very strong and predominate over thermal energy but in liquid, these forces are no longer strong enough.
(ii) Because of very weak intermolecular forces and high thermal energy, molecules of gases are far apart. That is why gases are highly compressible.
Question 5.
A gas is enclosed in room. The temperature, pressure, density and number of moles respectively are t°C,p atm, g cm-3 and n moles.
(i) What will be the pressure, temperature, density and number of moles in each compartment, if room is partitioned into four equal compartments?
(ii) What will be the value of pressure, temperature, density and number of moles in each compartment if the walls between the two compartments (say 1 and 2) are removed?
(iii) What will be the values of pressure, temperature, density and number of moles, if an equal volume of gas at pressure
(p) and temperature (t) is let inside the same room? .
Answer:
(i) (a) Pressure in each compartment is same, (p atm)
(b) Temperature will remain same (t°C).
(c) Density will remain same (d g cm-3).
(d) Because of partition, volume of each compartment becomes 1/4 and the number of molecules also become 1/4. The number of moles in each compartment will be n/4.
(ii) (a) Pressure will remain same (p atm).
(b) Temperature will remain same (t°C).
(c) Density will remain same (d g cm-3).
(d) The number of moles in each compartment will be n/2.
(iii) (a) Pressure will be doubled (2p atm).
(b) Temperature will remain same.
(c) Density will remain same (d g cm-3) ,
(d) Number of moles will be doubled i.e., 2n.
![]()
Long Answer Type Questions
Question 1.
Explain the following:
(i) The boiling point of a liquid rises on increasing pressure. ;
(ii) Drops of liquid assume spherical space.
(iii) The boiling point of water (373 K) is abnormally high when compared to that of H2S (211.2 K).
(iv) The level of mercury in capillary tube is lower than the s level outside when a capillary tube is inserted in the mercury.
(v) Tea or coffee is sipped from a saucer when it is quite hot.
Answer:
(i) A liquid boils when its vapour pressure becomes equal to the atmospheric pressure. An increase in pressure on liquid, therefore, causes a rise in the boiling temperature of the liquids.
(ii) Liquids have a property, called surface tension, due to which liquids tend to contract (to decrease the surface area). For a given volume of a liquid, since a sphere has the least surface area, hence the liquids tend to form spherical droplet.
(iii) The extensive hydrogen bonding in water gives a polymeric structure. This makes the escape of molecules from the liquid more difficult. Therefore, water requires higher temperature to bring its vapour pressure equal to the atmospheric pressure.
On the other hand, sulphur being less electronegative, does not form hydrogen bonds with H of H2S. As a result, H2S has low boiling point.
(iv) The cohesive forces in mercury are much stronger than the force of adhesion between glass and mercury. Therefore, mercury-glass contract angle is greater than 90°C.
As a result, the vertical component of the surface tension forces acts vertically downward, thereby lowering the level of mercury column in the capillary tube.
(v) Evaporation causes cooling and the rate of evaporation increases with an increase in the surface area. Since, saucer has a large surface area, hence tea/coffee taken in a saucer cools quickly.
![]()
Question 2.
Nitrogen molecule (N2) has radius of about 0.2 nm. Assuming that nitrogen molecule is spherical in shape, calculate
(i) volume of a single molecule of N2.
(ii) the percentage of empty space in one mole of N2 gas at STP.
Answer:
(i) The volume of a sphere = \(\frac{4}{3}\)πr3 nr where Volume of a molecule of N2
= \(\frac{4}{3} \times \frac{22}{7}\) x (2 x 10-8)3 cm3 – 3.35 x 10-23 cm3
(ii) To calculate the empty space, let us first find the total volume of 1 mole (6.022 x 1023 molecules) of N2.
Volume of 6.022 x 1023 molecules of N2
= 3.35 x 10-23 x 6.022 x 1023 = 20.17 cm3
Now, volume occupied by 1 mole of gas at STP
= 22.4 litre = 22400 cm3
Empty volume = Total volume of gas – Volume occupied by molecules
= (22400 – 20.17) cm3 – 22379.83 cm3
∴ Percentage of empty space = \(\frac{Empty space}{Total volume}\) x 100
= \(\frac{22379.83}{22400}\) x 100 = 99.9%
Thus, 99.9% of space of 1 mole of N2 at STP is empty.