This PSEB 10th Class Science Notes Chapter 5 Periodic Classification of Elements will help you in revision during exams.
PSEB 10th Class Science Notes Chapter 5 Periodic Classification of Elements
→ The arrangement of elements in such a manner that the elements having similar elements are grouped together whereas the elements having different properties are separated is called classification of elements.
→ According to Dobereiner Triads, the elements were arranged in order of increasing atomic masses and grouped into three elements such, that the elements had similar properties such that the atomic mass of the middle element was average of the other two elements.
→ The classification done by Dobereiner on the basis of triads was not applicable to classify all the elements.
→ In 1866, on the basis of the law of octaves, Newland classified elements till calcium whose atomic mass is 40.
→ Russian chemist, Mendeleev discovered periodic law which is famous by the name Mendeleev’s periodic law which states that the physical and chemical properties of the elements are the periodic functions of their atomic masses.
![]()
→ Mendeleev’s periodic table was arranged into periods and groups.
→ Horizontal rows are called periods and vertical columns are called groups.
→ In the main periodic table, Mendeleev left the places for those elements which were not discovered till then.
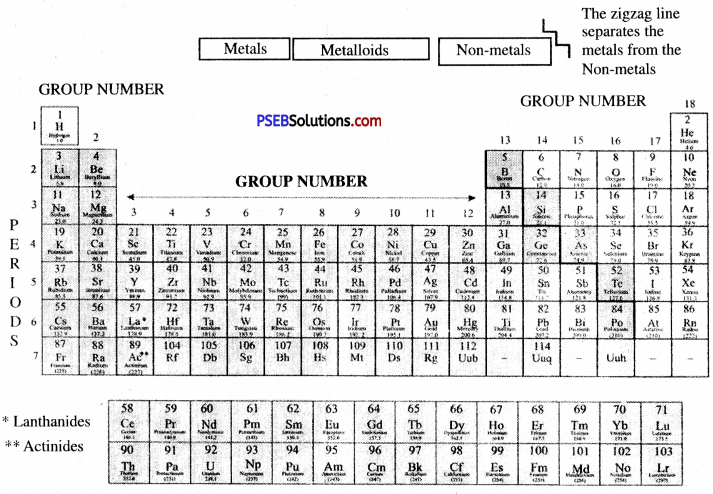
→ According to Modern Periodic Law, the physical and chemical properties of elements are the periodic functions of their atomic number.
→ In modern periodic tables, elements are arranged in 18 vertical columns known as groups and 7 horizontal rows known as periods.
→ Elements of group zero/18 are known as noble gases.
→ The repetition of the similar properties of elements placed in a group and separated by definite gaps of atomic number is called periodicity.
→ There are also some defects in the long form of the periodic table.
→ Scandium, gallium, germanium, etc., were discovered after Mendeleev’s periodic table. In 1913, Henry Moseley said that in comparison to the atomic mass of elements, atomic number is a more fundamental property.
→ By arranging the elements in ascending order of atomic number, their properties can be estimated more precisely.
→ By atomic size, we can find the atomic radius.
→ The atomic radius of Hydrogen is 37 pm (Picometer, 1 pm = HP12 m).
→ Atomic radius decreases on moving left to right along a period.
![]()
→ By metalloids properties of metal and non-metals are differentiated. Metalloids are-Boron, Silicon, Germanium,
→ Arsenic, Antimony, Tellurium, and Polonium. Oxides of metals are basic and oxides of non-metals are acidic.
→ The minimum energy required to remove an electron from the valence shell of an isolated gaseous atom is known as ionization energy.
→ The energy released when an electron enters the outermost shell of a neutral gaseous atom is known as electron affinity.
→ As we move down in a group, metallic properties increases.
→ On moving down in a group from top to bottom ionization energy decreases.
→ Periodic Table: This is a table in which we classify the elements in a specific order.
→ Newlands’ Law of Octaves: It states that when the elements are arranged in the ascending order of their increasing atomic weights, every eighth element has properties similar to the first element like the notes of an octave of music.
→ Dobereiner’s triads: It is a group of three elements having similar chemical properties in which the atomic weight of the middle element is the average of the other two elements.
→ Periodic classification is the systematic study of the properties of the elements.
→ Periodic Table: It is a table or chart in which the various elements are arranged in such a manner that elements having similar properties fall in the same vertical column whereas dissimilar elements are separated.
![]()
→ Mendeleev’s Periodic Table: The table of elements made by Mendeleev is known as Mendeleev’s Periodic Table, which is based on atomic mass.
→ Modern Periodic Table: The table which was made after the modification of Mendeleev’s Periodic Table, is known as Modern Periodic Table or extended form of the periodic table. It is based on atomic number.
→ Mendeleev’s Periodic Law: Physical and chemical properties of elements are the periodic functions of their atomic masses.
→ Periods: Horizontal rows in a periodic table are known as periods.
→ Groups: Vertical columns of the periodic table are known as groups.
→ Modern Periodic Law: The physical and chemical properties of elements are the periodic function of their atomic number.
→ Periodicity: Repetition of characteristics of elements in a group is known as periodicity.
→ Atomic Radius: The distance from the nucleus of an atom to its outer shell is known as atomic radius.
→ Valence Electrons: The number of electrons in the valence shell of an atom is known as valence electrons.
![]()
→ Ionization Potential: The ionization potential of an element is the minimum energy required to separate an electron from the outermost shell of a gaseous atom of that element.
→ Electron Affinity: The energy released when an electron enters the outermost shell of a neutral gaseous atom is known as electron affinity.
→ Valency is the combining capacity of the element and is equal to either the number of valence electrons or eight minus the number of valence electrons.
→ Lanthanides: Fourteen elements starting from lanthanum having atomic numbers 58 to 71 are called lanthanides.
→ Actinides: Fourteen elements starting from actinium having atomic numbers 90 to 103 are called actinides.
→ Metalloids: Elements that behave both as metals and non-metals are called metalloids.
→ Periodic Properties: These are the properties of an element that are related to the electronic configuration of its atom and change periodically down a group and along a period.
→ Atomic Size: In a period, with the increase of atomic number, there is a decrease in atomic radius. By going from left to right, the atomic number increases, and the size of the atom decreases. This is called atomic size.
→ Representative Elements: The elements of sub-group A are known as representative elements.
![]()
→ Need for classification: Elements are grouped based upon similarities in their properties in order to simply and systematically study of the properties of the elements.
Modern Periodic Table: